|
Isotopes Of Flerovium
Flerovium (114Fl) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 289Fl in 1999 (or possibly 1998). Flerovium has seven known isotopes, and possibly 2 nuclear isomers. The longest-lived isotope is 289Fl with a half-life of 1.9 seconds, but the unconfirmed 290Fl may have a longer half-life of 19 seconds. List of isotopes , - , 284Fl , style="text-align:right" , 114 , style="text-align:right" , 170 , , 3.1(13) ms , SF , (various) , 0+ , - , 285Fl , style="text-align:right" , 114 , style="text-align:right" , 171 , 285.18364(47)# , , α , 281Cn , 3/2+# , - , rowspan=2, 286Fl , rowspan=2 style="text-align:right" , 114 , rowspan=2 style="text-align:right" , 172 , rowspan=2, 286.18424(71)# , rowspan=2, , α (55%) , 282Cn , rowspan=2, 0+ , - , SF (45%) , (various) , - , rowspan=2, 287Fl , rowspan=2 style="text-align:righ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flerovium
Flerovium is a superheavy chemical element with symbol Fl and atomic number 114. It is an extremely radioactive synthetic element. It is named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia, where the element was discovered in 1998. The lab's name, in turn, honours Russian physicist Georgy Flyorov ( in Cyrillic, hence the transliteration of " yo" to "e"). IUPAC adopted the name on 30 May 2012. The name and symbol had previously been proposed for element 102 (nobelium), but was not accepted by IUPAC at that time. It is a transactinide in the p-block of the periodic table. It is in period 7; the heaviest known member of the carbon group, and the last element whose chemistry has been investigated. Initial chemical studies in 2007–2008 indicated that flerovium was unexpectedly volatile for a group 14 element; in preliminary results it even seemed to exhibit properties similar to noble gases. More recent results show ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copernicium
Copernicium is a synthetic chemical element with the symbol Cn and atomic number 112. Its known isotopes are extremely radioactive, and have only been created in a laboratory. The most stable known isotope, copernicium-285, has a half-life of approximately 28 seconds. Copernicium was first created in 1996 by the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany. It was named after the astronomer Nicolaus Copernicus. In the periodic table of the elements, copernicium is a d-block transactinide element and a group 12 element. During reactions with gold, it has been shown to be an extremely volatile element, so much so that it is possibly a gas or a volatile liquid at standard temperature and pressure. Copernicium is calculated to have several properties that differ from its lighter homologues in group 12, zinc, cadmium and mercury; due to relativistic effects, it may give up its 6d electrons instead of its 7s ones, and it may have more similarities to the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spontaneous Fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdown into smaller nuclei and a few isolated nuclear particles becomes possible at greater atomic mass numbers. History By 1908, physicists understood that alpha decay involved ejection of helium nuclei from a decaying atom. Like cluster decay, alpha decay is not typically categorized as a process of fission. The first nuclear fission process discovered was fission induced by neutrons. Because cosmic rays produce some neutrons, it was difficult to distinguish between induced and spontaneous events. Cosmic rays can be reliably shielded by a thick layer of rock or water. Spontaneous fission was identified in 1940 by Soviet physicists Georgy Flyorov and Konstantin Petrzhak by their observations of uranium in the Moscow Metro Dinamo station ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
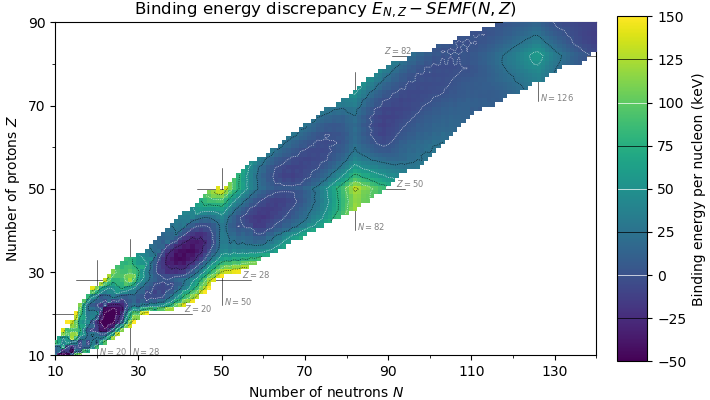
Doubly Magic
In nuclear physics, a magic number is a number of nucleons (either protons or neutrons, separately) such that they are arranged into complete Nuclear shell model, shells within the atomic nucleus. As a result, atomic nuclei with a 'magic' number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126 . For protons, this corresponds to the elements helium, oxygen, calcium, nickel, tin, lead and the hypothetical unbihexium, although 126 is so far only known to be a magic number for neutrons. Atomic nuclei consisting of such a magic number of nucleons have a higher average binding energy per nucleon than one would expect based upon predictions such as the semi-empirical mass formula and are hence more stable against nuclear decay. The unusual stability of isotopes having magic numbers means that transuranium elements could theoretically be created with extremely large nuclei and yet n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magic Number (physics)
In nuclear physics, a magic number is a number of nucleons (either protons or neutrons, separately) such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a 'magic' number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126 . For protons, this corresponds to the elements helium, oxygen, calcium, nickel, tin, lead and the hypothetical unbihexium, although 126 is so far only known to be a magic number for neutrons. Atomic nuclei consisting of such a magic number of nucleons have a higher average binding energy per nucleon than one would expect based upon predictions such as the semi-empirical mass formula and are hence more stable against nuclear decay. The unusual stability of isotopes having magic numbers means that transuranium elements could theoretically be created with extremely large nuclei and yet not be subject to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Review C
Physical may refer to: *Physical examination In a physical examination, medical examination, or clinical examination, a medical practitioner examines a patient for any possible medical signs or symptoms of a medical condition. It generally consists of a series of questions about the patien ..., a regular overall check-up with a doctor * ''Physical'' (Olivia Newton-John album), 1981 ** "Physical" (Olivia Newton-John song) * ''Physical'' (Gabe Gurnsey album) * "Physical" (Alcazar song) (2004) * "Physical" (Enrique Iglesias song) (2014) * "Physical" (Dua Lipa song) (2020) *"Physical (You're So)", a 1980 song by Adam & the Ants, the B side to " Dog Eat Dog" * ''Physical'' (TV series), an American television series See also {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Even Z Alpha Decay Chains
Even may refer to: General * Even (given name), a Norwegian male personal name * Even (surname) * Even (people), an ethnic group from Siberia and Russian Far East **Even language, a language spoken by the Evens * Odd and Even, a solitaire game which is played with two decks of playing cards Science and technology *In mathematics, the term ''even'' is used in several senses related to ''odd'': ** even and odd numbers, an integer is even if dividing by two yields an integer ** even and odd functions, a function is even if ''f''(−''x'') = ''f''(''x'') for all ''x'' ** even and odd permutations, a permutation of a finite set is even if it is composed of an even number of transpositions **Singly even number, an integer divisible by 2 but not divisible by 4 * Even code, if the Hamming weight of all of a binary code's codewords is even Entertainment *Even (band) Even are an Australian indie rock three-piece fronted by singer-songwriter-guitarist, Ashley Naylor with Matt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mean Lifetime
A quantity is subject to exponential decay if it decreases at a rate Proportionality (mathematics), proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda) is a positive rate called the exponential decay constant, disintegration constant, rate constant, or transformation constant: :\frac = -\lambda N. The solution to this equation (see #Solution_of_the_differential_equation, derivation below) is: :N(t) = N_0 e^, where is the quantity at time , is the initial quantity, that is, the quantity at time . Measuring rates of decay Mean lifetime If the decaying quantity, ''N''(''t''), is the number of discrete elements in a certain set (mathematics), set, it is possible to compute the average length of time that an element remains in the set. This is called the mean lifetime (or simply the lifetime), where the exponential time constant, \tau, relates to the decay rate constant, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oganesson
Oganesson is a synthetic chemical element with the symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint team of Russian and American scientists. In December 2015, it was recognized as one of four new elements by the Joint Working Party of the international scientific bodies IUPAC and IUPAP. It was formally named on 28 November 2016. The name honors the nuclear physicist Yuri Oganessian, who played a leading role in the discovery of the heaviest elements in the periodic table. It is one of only two elements named after a person who was alive at the time of naming, the other being seaborgium, and the only element whose eponym is alive today. Oganesson has the highest atomic number and highest atomic mass of all known elements. The radioactive oganesson atom is very unstable, and since 2005, only five (possibly six) atoms of the isotope oganesson-294 have been detected. Althou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Livermorium
Livermorium is a synthetic chemical element with the symbol Lv and has an atomic number of 116. It is an extremely radioactive element that has only been created in a laboratory setting and has not been observed in nature. The element is named after the Lawrence Livermore National Laboratory in the United States, which collaborated with the Joint Institute for Nuclear Research (JINR) in Dubna, Russia to discover livermorium during experiments conducted between 2000 and 2006. The name of the laboratory refers to the city of Livermore, California, where it is located, which in turn was named after the rancher and landowner Robert Livermore. The name was adopted by IUPAC on May 30, 2012. Four isotopes of livermorium are known, with mass numbers between 290 and 293 inclusive; the longest-lived among them is livermorium-293 with a half-life of about 60 milliseconds. A fifth possible isotope with mass number 294 has been reported but not yet confirmed. In the periodic table, it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Chain
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay directly to a stable state, but rather undergo a series of decays until eventually a stable isotope is reached. Decay stages are referred to by their relationship to previous or subsequent stages. A ''parent isotope'' is one that undergoes decay to form a ''daughter isotope''. One example of this is uranium (atomic number 92) decaying into thorium (atomic number 90). The daughter isotope may be stable or it may decay to form a daughter isotope of its own. The daughter of a daughter isotope is sometimes called a ''granddaughter isotope''. The time it takes for a single parent atom to decay to an atom of its daughter isotope can vary widely, not only between different parent-daughter pairs, but also randomly between identical pairings of parent a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


_Halflife_of_Radionulides_depending_on_Z²_to_A_ratio.png)