|
Copernicium
Copernicium is a synthetic chemical element; it has symbol Cn and atomic number 112. Its known isotopes are extremely radioactive, and have only been created in a laboratory. The most stable known isotope, copernicium-285, has a half-life of approximately 30 seconds. Copernicium was first created in February 1996 by the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany. It was named after the astronomer Nicolaus Copernicus on his 537th anniversary. In the periodic table of the elements, copernicium is a d-block transactinide element and a group 12 element. During reactions with gold, it has been shown to be an extremely volatile element, so much so that it is possibly a gas or a volatile liquid at standard temperature and pressure. Copernicium is calculated to have several properties that differ from its lighter homologues in group 12, zinc, cadmium and mercury; due to relativistic effects, it may give up its 6d electrons instead of its 7s ones, and i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 12 Element
Group 12, by modern IUPAC numbering, is a group of chemical elements in the periodic table. It includes zinc (Zn), cadmium (Cd), mercury (Hg), and copernicium (Cn). Formerly this group was named ''IIB'' (pronounced as "group two B", as the "II" is a Roman numeral) by CAS and old IUPAC system. The three group 12 elements that occur naturally are zinc, cadmium and mercury. They are all widely used in electric and electronic applications, as well as in various alloys. The first two members of the group share similar properties as they are solid metals under standard conditions. Mercury is the only metal that is known to be a liquid at room temperature – as copernicium's boiling point has not yet been measured accurately enough, it is not yet known whether it is a liquid or a gas under standard conditions. While zinc is very important in the biochemistry of living organisms, cadmium and mercury are both highly toxic. As copernicium does not occur in nature, it has to be synthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicolaus Copernicus
Nicolaus Copernicus (19 February 1473 – 24 May 1543) was a Renaissance polymath who formulated a mathematical model, model of Celestial spheres#Renaissance, the universe that placed heliocentrism, the Sun rather than Earth at its center. Copernicus likely developed his model independently of Aristarchus of Samos, an List of ancient Greek astronomers, ancient Greek astronomer who had formulated such a model some eighteen centuries earlier. The publication of Copernicus' model in his book ' (''On the Revolutions of the Celestial Spheres''), just before his death in 1543, was a major event in the history of science, triggering the Copernican Revolution and making a pioneering contribution to the Scientific Revolution. Copernicus was born and died in Royal Prussia, a semiautonomous and multilingual region created within the Crown of the Kingdom of Poland from lands regained from the Teutonic Order after the Thirteen Years' War (1454–1466), Thirteen Years' War. A Poly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discovery Of The Chemical Elements
The discoveries of the 118 chemical elements known to exist as of 2025 are presented here in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately determined. There are plans to synthesize more elements, and it is not known how many elements are possible. Each element's list of elements by name, name, atomic number, year of first report, name of the discoverer, and notes related to the discovery are listed. Periodic table of elements Graphical timeline ImageSize = width:1600 height:120 # barincrement:0 PlotArea = top:70 bottom:30 right:10 left:10 AlignBars = justify Colors = id:gray1 value:gray(0.85) legend:Independent id:gray2 value:gray(0.95) DateFormat = yyyy Period = from:1665 till:2025 TimeAxis = orientation:horizontal ScaleMajor = unit:year increment:10 start:1670 ScaleMinor = unit:year increment:1 start:1665 TextData = tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
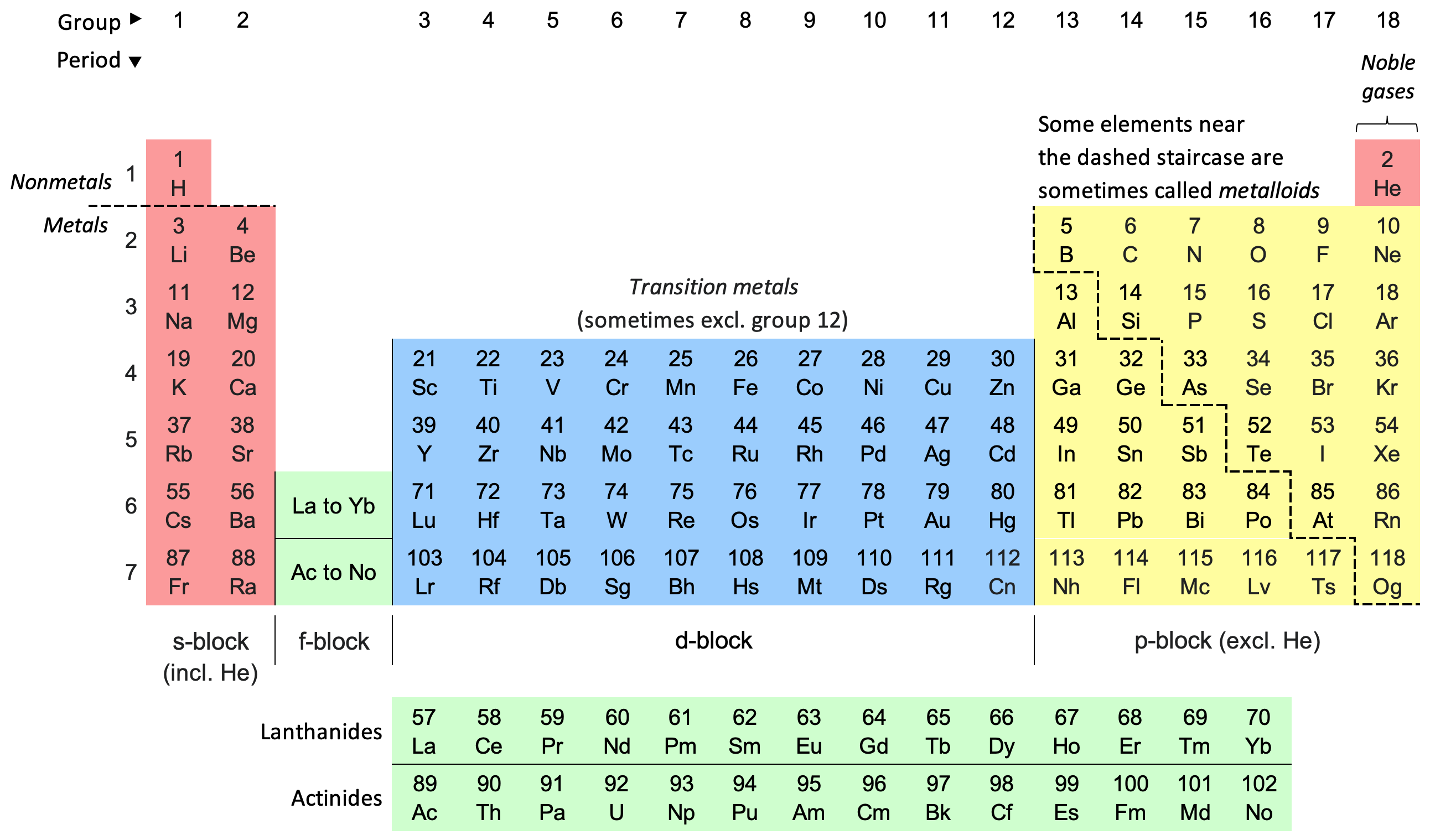
Periodic Table (standard)
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as a d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Symbol
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek language, Greek words. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (element), mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a Neo-Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter Systematic element name, temporary sym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transactinide Element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, or superheavies for short, are the chemical elements with atomic number greater than 104. The superheavy elements are those beyond the actinides in the periodic table; the last actinide is lawrencium (atomic number 103). By definition, superheavy elements are also transuranium elements, i.e., having atomic numbers greater than that of uranium (92). Depending on the definition of group 3 adopted by authors, lawrencium may also be included to complete the 6d series. Glenn T. Seaborg first proposed the actinide concept, which led to the acceptance of the actinide series. He also proposed a transactinide series ranging from element 104 to 121 and a superactinide series approximately spanning elements 122 to 153 (though more recent work suggests the end of the superactinide series to occur at element 157 instead). The transactinide seaborgium was named in his honor. Superheavies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury (element)
Mercury is a chemical element; it has Symbol (chemistry), symbol Hg and atomic number 80. It is commonly known as quicksilver. A Heavy metal element, heavy, silvery d-block element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by Mill (grinding), grinding natural cinnabar or synthetic mercuric sulfide. Exposure to mercury and mercury-containing organic compounds is toxic to the nervous system, immune system and kidneys of humans and other animals; mercury poisoning can result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury) either directly or through mechanisms of biomagnification. Mercu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is a known chemical element that does not occur naturally on Earth: it has been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, it is called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the Atomic nucleus, nucleus of an element with an atomic number lower than 95. All known (see: Island of stability) synthetic elements are unstable, but they radioactive decay, decay at widely varying rates; the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were first created artificially are strictly speaking not ''synthetic'' because they were later found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigurd Hofmann
Sigurd Hofmann (15 February 1944 – 17 June 2022) was a German physicist known for his work on superheavy elements. Biography Hofmann was born in Böhmisch Kamnitz, Nazi Germany (now Česká Kamenice, Czech Republic) on 15 February 1944. He discovered his love for physics at the Max Planck High School in Groß-Umstadt, Germany, where he graduated in 1963. He studied physics at the Technical University in Darmstadt (Diploma, 1969, and thesis at the Institute of Nuclear Physics with Egbert Kankeleit and Karl Wien, 1974). From 1974 to 1989 he was responsible for the detection and identification of nuclei produced in heavy ion reactions at the velocity separator SHIP (Separator for Heavy Ion reaction Products) at the GSI Helmholtz Centre for Heavy Ion Research. He was working in the Department Nuclear Chemistry II headed by Peter Armbruster. From 1989 he was leading, after Gottfried Münzenberg, the experiments for the synthesis of new elements. From 1998 he was Honorary Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gesellschaft Für Schwerionenforschung
The GSI Helmholtz Centre for Heavy Ion Research () is a federally and state co-funded heavy ion () research center in Darmstadt, Germany. It was founded in 1969 as the Society for Heavy Ion Research (), abbreviated GSI, to conduct research on and with heavy-ion accelerators. It is the only major user research center in the State of Hesse. The laboratory performs basic and applied research in physics and related natural science disciplines. Main fields of study include plasma physics, atomic physics, nuclear structure and reactions research, biophysics and medical research. The lab is a member of the Helmholtz Association of German Research Centres. Shareholders are the German Federal Government (90%) and the State of Hesse, Thuringia and Rhineland-Palatinate. As a member of the Helmholtz Association, the current name was given to the facility on 7 October 2008 in order to bring it sharper national and international awareness. The GSI Helmholtz Centre for Heavy Ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(IV) Fluoride
Mercury(IV) fluoride, HgF4, is a purported compound, the first to be reported with mercury in the +4 oxidation state. Mercury, like the other group 12 elements (cadmium and zinc), has an s2d10 electron configuration and generally only forms bonds involving its 6s orbital. This means that the highest oxidation state mercury normally attains is +2, and for this reason it is sometimes considered a post-transition metal instead of a transition metal. HgF4 was first reported from experiments in 2007, but its existence remains disputed; experiments conducted in 2008 could not replicate the compound. History Speculation about higher oxidation states for mercury had existed since the 1970s, and theoretical calculations in the 1990s predicted that it should be stable in the gas phase, with a square-planar geometry consistent with a formal d8 configuration. However, experimental proof remained elusive until 2007, when HgF4 was first prepared using solid neon and argon for matrix ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Victor Ninov
Victor Ninov (; born June 27, 1959) is a Bulgarian physicist and former researcher who worked primarily in creating superheavy elements. He is known for the co-discoveries of elements 110, 111, and 112 ( darmstadtium, roentgenium and copernicium, respectively). Ninov also claimed the creation of elements 116 and 118 (now livermorium and oganesson); however, an investigation conducted by the University of California, Berkeley concluded that he had falsified the evidence. The repercussions of the affair had an impact on the guidelines of conduct for several research institutions. Early life Victor Ninov was born in Bulgaria in 1959. He grew up in the capital city of Sofia. In the 1970s, when Ninov was a teenager, he and his family left for West Germany; they bounced around from house to house. Shortly after the move, Victor's father went missing; he was found dead six months later in the Bulgarian foothills due to causes unknown. Career Victor Ninov attended Technische ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |