|
Iron-binding Proteins
Iron-binding proteins are carrier proteins and metalloproteins that are important in iron metabolism and the immune response. Iron is required for life. Iron-dependent enzymes catalyze a variety of biochemical reactions and can be divided into three broad classes depending on the structure of their active site: non-heme mono-iron, non-heme diiron , or heme centers. A well-known family of iron-dependent enzymes include oxygenases that facilitate hydroxyl group addition of one or both atoms from o2. Notable enzymes include tryptophan dioxygenase, ferredoxin, and 2-oxoglutarate dioxygenase. Heme proteins Heme proteins are proteins that contain a heme prosthetic group. The heme group consists of a porphyrin ring coordinated with an iron ion. Four nitrogen atoms in the porphyrin ring act as a ligand for the iron in the center. In many cases, the equatorial porphyrin is complemented by one or two axial ligands. An example of this is in hemoglobin, where the porphyrin works together ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carrier Protein
A membrane transport protein (or simply transporter) is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion or active transport. The two main types of proteins involved in such transport are broadly categorized as either ''channels'' or ''carriers''. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans. Collectively membrane transporters and channels are known as the transportome. Transportomes govern cellular influx and efflux of not only ions and nutrients but drugs as well. Difference between channels and carriers A carrier is not open simultaneously to both the extracellular and intracellular environments. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins. Abundance It is estimated that approximately half of all proteins contain a metal. In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions. Thus, metalloproteins have many different functions in cells, such as storage and transport of proteins, enzymes and signal transduction proteins, or infectious diseases. The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals. Most metals in the human body are bound to proteins. For instance, the relatively high concentration of iron in the human body ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism. Metabolic reactions may be categorized as '' catabolic'' – the ''breaking down'' of compounds (for example, of glucose to pyruvate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic. One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from the Greek word πορφύρα (''porphyra''), meaning ''purple''. Complexes of porphyrins Concomitant with the displacement of two N-''H'' protons, porphyrins bind metal ions in the N4 "pocket". The metal ion usually has a charge of 2+ or 3+. A schematic equation for these syntheses is shown: :H2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Molecular Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix ''octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin T-r State Ani
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide Poisoning
Carbon monoxide poisoning typically occurs from breathing in carbon monoxide (CO) at excessive levels. Symptoms are often described as " flu-like" and commonly include headache, dizziness, weakness, vomiting, chest pain, and confusion. Large exposures can result in loss of consciousness, arrhythmias, seizures, or death. The classically described "cherry red skin" rarely occurs. Long-term complications may include chronic fatigue, trouble with memory, and movement problems. CO is a colorless and odorless gas which is initially non-irritating. It is produced during incomplete burning of organic matter. This can occur from motor vehicles, heaters, or cooking equipment that run on carbon-based fuels. Carbon monoxide primarily causes adverse effects by combining with hemoglobin to form carboxyhemoglobin (HbCO) preventing the blood from carrying oxygen and expelling carbon dioxide as carbaminohemoglobin. Additionally, many other hemoproteins such as myoglobin, Cytochrome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein TF PDB 1a8e
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
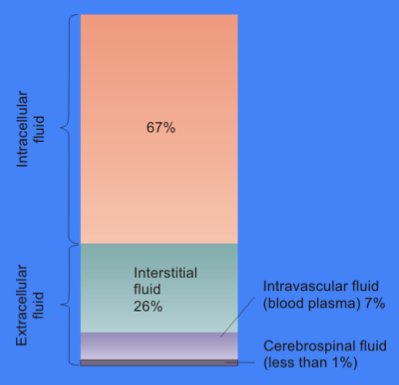
Cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into many compartments. In the eukaryotic cell, the cytosol is surrounded by the cell membrane and is part of the cytoplasm, which also comprises the mitochondria, plastids, and other organelles (but not their internal fluids and structures); the cell nucleus is separate. The cytosol is thus a liquid matrix around the organelles. In prokaryotes, most of the chemical reactions of metabolism take place in the cytosol, while a few take place in membranes or in the periplasmic space. In eukaryotes, while many metabolic pathways still occur in the cytosol, others take place within organelles. The cytosol is a complex mixture of substances dissolved in water. Although water forms the large majority of the cytosol, its structure and prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferrin Receptor
Transferrin receptor (TfR) is a carrier protein for transferrin. It is needed for the import of iron into the cell and is regulated in response to intracellular iron concentration. It imports iron by internalizing the transferrin-iron complex through receptor-mediated endocytosis.Figure 3: The cycle of transferrin and transferrin receptor 1-mediated cellular iron uptake./ref> The existence of a receptor for transferrin iron uptake had been recognized over half a century back. Earlier two transferrin receptors in humans, transferrin receptor 1 and transferrin receptor 2 had been characterized and until recently cellular iron uptake was believed to occur chiefly via these two well documented transferrin receptors. Both these receptors are transmembrane glycoproteins. TfR1 is a high affinity ubiquitously expressed receptor while expression of TfR2 is restricted to certain cell types and is unaffected by intracellular iron concentrations. TfR2 binds to transferrin with a 25-30 fold low ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





.jpg)