|
Ionic Atmosphere
Ionic Atmosphere is a concept employed in Debye-Hückel theory which explains the electrolytic conductivity behaviour of solutions. It can be generally defined as the area at which a charged entity is capable of attracting an entity of the opposite charge. Asymmetry, or relaxation effect If an electrical potential is applied to an electrolytic solution, a positive ion will move towards the negative electrode and drag along an entourage of negative ions with it. The more concentrated the solution, the closer these negative ions are to the positive ion and thus the greater the resistance experienced by the positive ion. This influence on the speed of an ion is known as the "Asymmetry effect" because the ionic atmosphere moving around the ion is not symmetrical; the charge density behind is greater than in the front, slowing the motion of the ion. Laidler K.J. and Meiser J.H., ''Physical Chemistry'' (Benjamin/Cummings 1982) p.269 The time required to form a new ionic atmosphere o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolytic Conductivity
Conductivity (or specific conductance) of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is Siemens per meter (S/m). Conductivity measurements are used routinely in many industrial and environmental applications as a fast, inexpensive and reliable way of measuring the ionic content in a solution. For example, the measurement of product conductivity is a typical way to monitor and continuously trend the performance of water purification systems. In many cases, conductivity is linked directly to the total dissolved solids (TDS). High quality deionized water has a conductivity of about 0.05 μS/cm at 25 °C, typical drinking water is in the range of 200–800 μS/cm, while sea water is about 50 mS/cm ncorrect according to source(or 50,000 μS/cm). Conductivity is traditionally determined by connecting the electrolyte in a Wheatstone bridge. Dilute solutions follow Kohlrausch's Laws of concent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keith J
Keith may refer to: People and fictional characters * Keith (given name), includes a list of people and fictional characters * Keith (surname) * Keith (singer), American singer James Keefer (born 1949) * Baron Keith, a line of Scottish barons in the late 18th century * Clan Keith, a Scottish clan associated with lands in northeastern and northwestern Scotland Places Australia * Keith, South Australia, a town and locality Scotland * Keith, Moray, a town ** Keith railway station * Keith Marischal, East Lothian United States * Keith, Georgia, an unincorporated community * Keith, Ohio, an unincorporated community * Keith, West Virginia, an unincorporated community * Keith, Wisconsin, a ghost town * Keith County, Nebraska Other uses * Keith F.C., a football team based in Keith, Scotland * , a ship of the British Royal Navy * Hurricane Keith, a 2000 hurricane that caused extensive damage in Central America * ''Keith'' (film), a 2008 independent film directed by Todd Kessle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye Falkenhagen Effect
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is given as charge times length of separation, it is a vector whose direction is in the direction of the unit vector of the position vector of the positive charge w.r.t negative charge: :p = ''q''r. named in honour of the physicist Peter J. W. Debye. It is defined as statcoulomb- centimeters.The statcoulomb is also known as the franklin or electrostatic unit of charge. :1 statC = 1 Fr = 1 esu = 1 cm3/2⋅g1/2⋅s−1. Historically the debye was defined as the dipole moment resulting from two charges of opposite sign but an equal magnitude of 10−10 statcoulomb10−10 statcoulomb corresponds to approximately 0.2083 units of elementary charge. (generally called e.s.u. (electrostatic unit) in older scientif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High Frequency
High frequency (HF) is the International Telecommunication Union, ITU designation for the range of radio frequency electromagnetic waves (radio waves) between 3 and 30 megahertz (MHz). It is also known as the decameter band or decameter wave as its wavelengths range from one to ten decametre, decameters (ten to one hundred meters). Frequencies immediately below HF are denoted medium frequency (MF), while the next band of higher frequencies is known as the very high frequency (VHF) band. The HF band is a major part of the shortwave band of frequencies, so communication at these frequencies is often called shortwave radio. Because radio waves in this band can be reflected back to Earth by the ionosphere layer in the atmosphere – a method known as "skip" or "skywave" propagation – these frequencies are suitable for long-distance communication across intercontinental distances and for mountainous terrains which prevent Line-of-sight propagation, line-of-sight communica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic Organic may refer to: * Organic, of or relating to an organism, a living entity * Organic, of or relating to an anatomical organ Chemistry * Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ... solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners ( toluene, turpentine); as nail polish removers and solvents of glue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molten Salt
Molten salt is salt which is solid at standard temperature and pressure but enters the liquid phase due to elevated temperature. Regular table salt has a melting point of 801 °C (1474°F) and a heat of fusion of 520 J/g.Proc. Roy. Soc. Bibliography C.F. Baes, ''The chemistry and thermodynamics of molten salt reactor fuels'', Proc. AIME Nuclear Fuel Reprocessing Symposium, Ames, Iowa, USA, 1969 (August 25) {{Authority control Energy storage Metallurgical processes Inorganic solvents Ionic liquids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
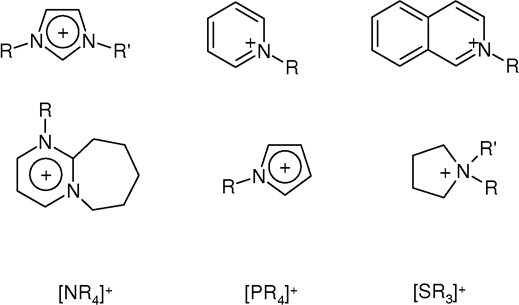
Ionic Liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many potential applications. They are powerful solvents and can be used as electrolytes. Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure. Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at into a liquid that consists largely of sodium cations () and chloride anions (). Conversely, when an ionic liquid is cooled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystalline Lattice
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Chemistry
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration. Analytical chemistry consists of classical, wet chemical methods and modern, instrumental methods. Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Instrumental methods may be used to separate samples using chromatography, electrophoresis or field flow fractionation. Then qualitative and quantitative analysis can be performed, often with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |