|
Internal Conversion
Internal conversion is an atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal conversion (often abbreviated IC), a high-energy electron is emitted from the excited atom, but not from the nucleus. For this reason, the high-speed electrons resulting from internal conversion are not called beta particles, since the latter come from beta decay, where they are newly created in the nuclear decay process. IC is possible whenever gamma decay is possible, except if the atom is fully ionized. In IC, the atomic number does not change, and thus there is no transmutation of one element to another. Also, neutrinos and the weak force are not involved in IC. Since an electron is lost from the atom, a hole appears in an electron aura which is subsequently filled by other electrons that descend to the empty, yet lower energy level, and in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester based on the 1909 Geiger–Marsden experiments, Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Scheme Of 203Hg
Decay may refer to: Science and technology * Bit decay, in computing * Decay time (fall time), in electronics * Distance decay, in geography * Software decay, in computing Biology * Decomposition of organic matter * Mitochondrial decay, in genetics * Tooth decay (dental caries), in dentistry Physics * Optical decay, in quantum physics * Orbital decay, the process of prolonged reduction in the height of a satellite's orbit * Particle decay * Radioactive decay * False vacuum decay Mathematics * Exponential decay Psychology and sociology * Decay theory, in psychology and memory * Social decay (decadence), in sociology * Urban decay, in sociology Entertainment * Network decay (channel drift), in television programming * Decay (DC Comics), a comic book character * '' Half-Life: Decay'', a 2001 video game add-on * Deekay, a Danish production team * Decay (professional wrestling), a professional wrestling stable in TNA Wrestling Film * ''Decay'' (2012 film), a 2012 zombie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Conversion Coefficient
In nuclear physics, the internal conversion coefficient describes the rate of internal conversion. The internal conversion coefficient may be empirically determined by the following formula: \alpha = \frac There is no valid formulation for an equivalent concept for E0 (electric monopole) nuclear transitions. There are theoretical calculations that can be used to derive internal conversion coefficients. Their accuracy is not generally under dispute, but since the quantum mechanical models they depend on only take into account electromagnetic interactions between the nucleus and electrons, there may be unforeseen effects. Internal conversion coefficients can be looked up from tables, but this is time-consuming. Computer programs have been developed (see thBrIcc Program which present internal conversion coefficients quickly and easily. Theoretical calculations of interest are the Rösel, Hager-Seltzer, and the Band, superseded by the Band-Raman calculation called BrIcc. The Hager ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ranging from 10 Nanometre, nanometers to 10 Picometre, picometers, corresponding to frequency, frequencies in the range of 30 Hertz, petahertz to 30 Hertz, exahertz ( to ) and photon energies in the range of 100 electronvolt, eV to 100 keV, respectively. X-rays were discovered in 1895 in science, 1895 by the German scientist Wilhelm Röntgen, Wilhelm Conrad Röntgen, who named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . X-rays can penetrate many solid substances such as construction materials and living tissue, so X-ray radiography is widely used in medical diagnostics (e.g., checking for Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoelectric Effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, solid state, and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission. The experimental results disagree with classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which would then be emitted when they accumulate enough energy. An alteration in the intensity of light would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in a delayed emission. The experimental results instead show that electrons are dislodged only when the light exceeds a certain frequency—regardless of the ligh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pair Production
Pair production is the creation of a subatomic particle and its antiparticle from a neutral boson. Examples include creating an electron and a positron, a muon and an antimuon, or a proton and an antiproton. Pair production often refers specifically to a photon creating an electron–positron pair near a nucleus. As energy must be conserved, for pair production to occur, the incoming energy of the photon must be above a threshold of at least the total rest mass energy of the two particles created. (As the electron is the lightest, hence, lowest mass/energy, elementary particle, it requires the least energetic photons of all possible pair-production processes.) Conservation of energy and momentum are the principal constraints on the process. All other conserved quantum numbers ( angular momentum, electric charge, lepton number) of the produced particles must sum to zero thus the created particles shall have opposite values of each other. For instance, if one partic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-125
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129. Its half-life is 59.392 days and it decays by electron capture to an excited state of tellurium-125. This state is not the metastable 125mTe, but rather a lower energy state. The excited 125Te may (7% chance) undergo gamma decay with a maximum energy of 35 keV. More often (93% chance), the excited 125Te undergoes internally conversion and ejects an electron (< 35 keV). The resulting electron vacancy leads to emission of characteristic X-rays (27–32 keV) and a total of 21 [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parity (physics)
In physics, a parity transformation (also called parity inversion) is the flip in the sign of ''one'' spatial coordinate. In three dimensions, it can also refer to the simultaneous flip in the sign of all three spatial coordinates (a point reflection): \mathbf: \beginx\\y\\z\end \mapsto \begin-x\\-y\\-z\end. It can also be thought of as a test for chirality of a physical phenomenon, in that a parity inversion transforms a phenomenon into its mirror image. All fundamental interactions of elementary particles, with the exception of the weak interaction, are symmetric under parity transformation. As established by the Wu experiment conducted at the US National Bureau of Standards by Chinese-American scientist Chien-Shiung Wu, the weak interaction is chiral and thus provides a means for probing chirality in physics. In her experiment, Wu took advantage of the controlling role of weak interactions in radioactive decay of atomic isotopes to establish the chirality of the weak f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
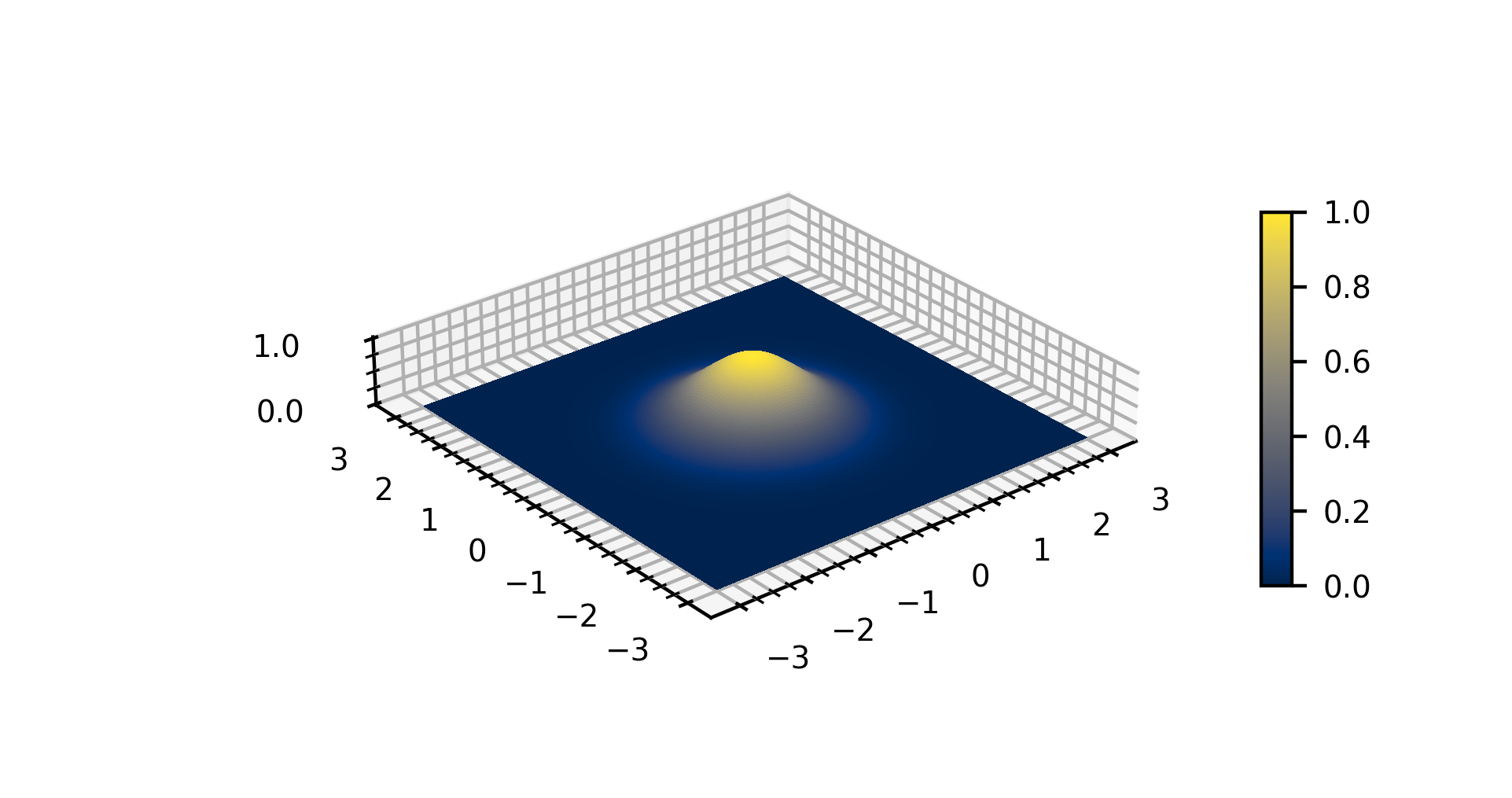
Gaussian Function
In mathematics, a Gaussian function, often simply referred to as a Gaussian, is a function (mathematics), function of the base form f(x) = \exp (-x^2) and with parametric extension f(x) = a \exp\left( -\frac \right) for arbitrary real number, real constants , and non-zero . It is named after the mathematician Carl Friedrich Gauss. The graph of a function, graph of a Gaussian is a characteristic symmetric "Normal distribution, bell curve" shape. The parameter is the height of the curve's peak, is the position of the center of the peak, and (the standard deviation, sometimes called the Gaussian Root mean square, RMS width) controls the width of the "bell". Gaussian functions are often used to represent the probability density function of a normal distribution, normally distributed random variable with expected value and variance . In this case, the Gaussian is of the form g(x) = \frac \exp\left( -\frac \frac \right). Gaussian functions are widely used in statistics to describ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrometer
A spectrometer () is a scientific instrument used to separate and measure Spectrum, spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed. In visible light a spectrometer can separate white light and measure individual narrow bands of color, called a spectrum. A mass spectrometer measures the spectrum of the masses of the atoms or molecules present in a gas. The first spectrometers were used to split light into an array of separate colors. Spectrometers were History_of_spectroscopy, developed in early studies of physics, astronomy, and chemistry. The capability of spectroscopy to determine Analytical_chemistry#Spectroscopy, chemical composition drove its advancement and continues to be one of its primary uses. Spectrometers are used in Astronomical spectroscopy, astronomy to analyze the chemical composition of Astronomical_spe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |