|
Immutep
Immutep Ltd (formerly Prima Biomed) is a biotechnology company working primarily in the field of cancer immunotherapy using the LAG3 immune control mechanism. The company was originally built on CVac, a therapeutic cancer vaccine. In late 2014 the privately held French immunotherapy company Immutep SA was purchased by Prima Biotech. Prima currently has three main products in its pipeline, all acquired with Immutep: Eftilagimod alpha, (lab name: IMP321) which is recombinant soluble LAG-3, used as an activator of antigen presenting cells. This product has completed a Phase IIa clinical study, where it doubled the expected response rate in HER2-negative metastatic breast cancer. IMP731, a depleting monoclonal antibody for autoimmune diseases, targeting LAG-3+ activated T cells. This antibody has been licensed to GlaxoSmithKline. IMP701, an antagonist monoclonal antibody to LAG3 for use in cancer. This product has been licensed to Novartis History Immutep (formerly Prima B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frédéric Triebel
Frédéric Triebel (born 20 November 1954) is a French immunologist who is best known for his 1990 discovery of the LAG3 immune control mechanism. Triebel worked through the 1990s in a collaboration between Institut Gustave Roussy and Merck Serono to establish LAG-3's mechanism of action in T cells and dendritic cells. In 2001 he founded Immutep SA, a biotech company, to develop the therapeutic potential of LAG3. In 2014 this company was acquired by Prima BioMed, where Triebel remains Chief Scientific and Medical Officer. Early life and education He completed his Doctor of Medicine degree at Poitiers University in 1981 and then a four-year clinical hematology fellowship in Paris hospitals. In 1983, Frédéric Triebel received the Gold Medal of the Paris Medicine University. In parallel, Triebel gained a PhD in Immunology at the University of Paris VI in 1985. His PhD thesis was in the field of immunogenetics, focused on the mechanisms that activate human antigen-specific T-cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eftilagimod Alpha
Eftilagimod alpha (INN; development code IMP321 or efti) is a large-molecule cancer drug being developed by the clinical-stage biotechnology company Immutep. Efti is a soluble version of the immune checkpoint molecule LAG-3. It is an APC Activator used to increase an immune response to tumors, and is administered by subcutaneous injection. Efti has three intended clinical settings: * as adjuvant to cancer vaccines (in a low, effective dose of ~250 µg) * as first-line 'chemo-immunotherapy,' that is, combined with standard chemotherapy (e.g. paclitaxel) * in combination immunotherapy with PD-1 treatments (e.g. pembrolizumab) Eftilagimod alpha is in Phase II clinical testing. Currently, the main indications for the drug are metastatic breast cancer, non-small cell lung cancer (NSCLC), and head and neck squamous cell carcinoma (HNSCC). Background Eftilagimod alpha ("efti" in short) is a soluble LAG-3 fusion protein that activates antigen-presenting cells. It is a 160 k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IMP321
Eftilagimod alpha (International Nonproprietary Name, INN; development code IMP321 or efti) is a large molecule, large-molecule cancer drug being developed by the clinical-stage biotechnology company Immutep. Efti is a soluble version of the immune checkpoint molecule LAG3, LAG-3. It is an APC Activator used to increase an immune response to tumors, and is administered by subcutaneous injection. Efti has three intended clinical settings: * as adjuvant to cancer vaccines (in a low, effective dose of ~250 µg) * as Therapy, first-line 'chemo-immunotherapy,' that is, combined with standard chemotherapy (e.g. paclitaxel) * in combination immunotherapy with PD-1 treatments (e.g. pembrolizumab) Eftilagimod alpha is in Phase II clinical testing. Currently, the main indications for the drug are metastatic breast cancer, Non-small-cell lung carcinoma, non-small cell lung cancer (NSCLC), and Head and neck cancer, head and neck squamous cell carcinoma (HNSCC). Background Eftilagimod a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GSK2831781
GSK2831781 is a monoclonal antibody being developed by GlaxoSmithKline (GSK) for autoimmune diseases. The antibody targets the T cell activation marker LAG-3, which is mainly expressed in inflamed tissues. In GSK's March 2015 ''Product development pipeline'' document the antibody is listed under 'Immuno-inflammation' candidates. GSK2831781 entered a Phase I clinical trial in psoriasis early in 2015. History GSK2831781 originated from a chimeric monoclonal antibody to LAG-3 developed in 2008 by the French biotechnology company Immutep. That company had been built around drugs targeting LAG-3 and was associated with Frédéric Triebel, an immunologist generally regarded as a leading authority on LAG-3. In discovering the Immutep antibody, Triebel worked with two researchers from the University of Nantes, where there was an INSERM unit focused on transplantation immunology called ITUN (Institut de Transplantation Urologie Nephrologie). Triebel et al. codenamed their initial m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LAG3
Lymphocyte-activation gene 3, also known as LAG-3, is a protein which in humans is encoded by the ''LAG3'' gene. LAG3, which was discovered in 1990 and was designated CD223 (cluster of differentiation 223) after the Seventh Human Leucocyte Differentiation Antigen Workshop in 2000, is a cell surface molecule with diverse biologic effects on T cell function. It is an immune checkpoint receptor and as such is the target of various drug development programs by pharmaceutical companies seeking to develop new treatments for cancer and autoimmune disorders. In soluble form it is also being developed as a cancer drug in its own right. Gene The LAG3 gene contains 8 exons. The sequence data, exon/ intron organization, and chromosomal localization all indicate a close relationship of LAG3 to CD4. The gene for LAG-3 lies adjacent to the gene for CD4 on human chromosome 12 (12p13) and is approximately 20% identical to the CD4 gene. Protein The LAG3 protein, which belongs to immunoglob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CVac
CVac (short for Cancer Vaccine), is an immunotherapeutic agent that was first developed in Australia. CVac was developed by the biotechnology company Prima BioMed (Nasdaq: PBMD) through to Phase II clinical studies between 2001 and 2015. After inconclusive clinical data in ovarian cancer 2013 and 2014, Prima announced in February 2015 that it had ceased recruiting into CVAc clinical studies and was seeking commercial partners for the product. Treatment method CVac is an '' ex vivo'' dendritic cell priming technology involving MFP, or mannan fusion protein, where a fusion protein of MUC1 and Glutathione S-transferase is coupled to oxidized polymannose. The dendritic cells are the orchestrators of the immune system. 'Priming' dendritic cells involves exposing those cells to disease antigens in order to get the immune system to properly recognise the antigen and mount an appropriate response. With CVac this takes place outside the body of the patient (''ex vivo''), with dendritic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P Value
In null-hypothesis significance testing, the ''p''-value is the probability of obtaining test results at least as extreme as the result actually observed, under the assumption that the null hypothesis is correct. A very small ''p''-value means that such an extreme observed outcome would be very unlikely under the null hypothesis. Reporting ''p''-values of statistical tests is common practice in academic publications of many quantitative fields. Since the precise meaning of ''p''-value is hard to grasp, misuse is widespread and has been a major topic in metascience. Basic concepts In statistics, every conjecture concerning the unknown probability distribution of a collection of random variables representing the observed data X in some study is called a ''statistical hypothesis''. If we state one hypothesis only and the aim of the statistical test is to see whether this hypothesis is tenable, but not to investigate other specific hypotheses, then such a test is called a null ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
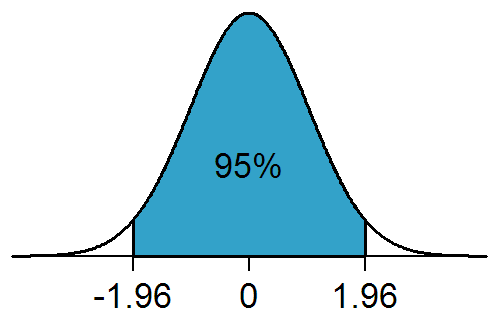
Statistical Significance
In statistical hypothesis testing, a result has statistical significance when it is very unlikely to have occurred given the null hypothesis (simply by chance alone). More precisely, a study's defined significance level, denoted by \alpha, is the probability of the study rejecting the null hypothesis, given that the null hypothesis is true; and the ''p''-value of a result, ''p'', is the probability of obtaining a result at least as extreme, given that the null hypothesis is true. The result is statistically significant, by the standards of the study, when p \le \alpha. The significance level for a study is chosen before data collection, and is typically set to 5% or much lower—depending on the field of study. In any experiment or observation that involves drawing a sample from a population, there is always the possibility that an observed effect would have occurred due to sampling error alone. But if the ''p''-value of an observed effect is less than (or equal to) the significanc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune Checkpoint
Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately. However, some cancers can protect themselves from attack by stimulating immune checkpoint targets. Inhibitory checkpoint molecules are targets for cancer immunotherapy due to their potential for use in multiple types of cancers. Currently approved checkpoint inhibitors block CTLA4 and PD-1 and PD-L1. For the related basic science discoveries, James P. Allison and Tasuku Honjo won the Tang Prize in Biopharmaceutical Science and the Nobel Prize in Physiology or Medicine in 2018. Stimulatory checkpoint molecules Four stimulatory checkpoint molecules are members of the tumor necrosis factor (TNF) receptor superfamily—CD27, CD40, OX40, GITR and CD137. Another two stimulatory checkpoint molecules belong to the B7-CD28 superfamily—CD28 itself and ICOS. * CD27: This molecule supports antigen-specific ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Progression-free Survival
Progression-free survival (PFS) is "the length of time during and after the treatment of a disease, such as cancer, that a patient lives with the disease but it does not get worse". In oncology, PFS usually refers to situations in which a tumor is present, as demonstrated by laboratory testing, radiologic testing, or clinically. Similarly, "disease-free survival" is the length of time after patients have received treatment and have no detectable disease. Time to progression (TTP) does not count patients who die from other causes but is otherwise a close equivalent to PFS (unless there are many such events). The FDA gives separate definitions and prefers PFS. Background PFS is widely used as a surrogate endpoint in oncology. The definition of "progression" generally involves imaging techniques (plain radiograms, CT scans, MRI, PET scans, ultrasounds) or other aspects: biochemical progression may be defined on the basis of an increase in a tumor marker (such as CA125 for epithelial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Institut Gustave Roussy
Gustave Roussy is the first leader cancer-research hospital in Europe and ranked among the top 3 best specialized hospitals in the world . It is a centre for high quality patient care, research and teaching. It is highly-known for the treatment of (among others): skin cancers like melanoma, breast cancer, and lung cancer. It provides access to care with many expert doctors who have historically revolutionized the treatment of cancer and contributed to the surge of new molecules in the treatment of cancers and tumors. It is located in the Parisian area. It is named after Gustave Roussy, a Swiss-French neuropathologist. In April 2019, three new interventional radiology rooms were inaugurated, making it the largest platform of this type in Europe, entirely dedicated to oncology. Interventional radiology is a so-called "minimally invasive" diagnostic and treatment technique, which uses images to guide access to deep-lying organs, without having to "open up" patients. Gustave Roussy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GlaxoSmithKline
GSK plc, formerly GlaxoSmithKline plc, is a British multinational pharmaceutical and biotechnology company with global headquarters in London, England. Established in 2000 by a merger of Glaxo Wellcome and SmithKline Beecham. GSK is the tenth largest pharmaceutical company and #294 on the 2022 ''Fortune'' Global 500, ranked behind other pharmaceutical companies China Resources, Sinopharm, Johnson & Johnson, Pfizer, Roche, AbbVie, Novartis, Bayer, and Merck. The company has a primary listing on the London Stock Exchange and is a constituent of the FTSE 100 Index. , it had a market capitalisation of £70 billion, the eighth largest on the London Stock Exchange. It has a secondary listing on the New York Stock Exchange. The company developed the first malaria vaccine, RTS,S, which it said in 2014 it would make available for five percent above cost. Legacy products developed at GSK include several listed in the World Health Organization's List of Essential Medicines, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |