|
ICAM3
Intercellular adhesion molecule 3 (ICAM3) also known as CD50 (Cluster of Differentiation 50), is a protein that in humans is encoded by the ''ICAM3'' gene. The protein is constitutively expressed on the surface of leukocytes, which are also called white blood cells and are part of the immune system. ICAM3 mediates adhesion between cells by binding to specific integrin receptors. It plays an important role in the immune cell response through its facilitation of interactions between T cells and dendritic cells, which allows for T cell activation. ICAM3 also mediates the clearance of cells undergoing apoptosis by attracting and binding macrophages, a type of cell that breaks down infected or dying cells through a process known as phagocytosis, to apoptotic cells. Protein Structure ICAM3 is a 110-160 kDa protein that belongs to the intercellular adhesion molecule (ICAM) family. Like the other proteins in this family, ICAM3 is a type I transmembrane glycoprotein and consists in part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moesin
Moesin is a protein that in humans is encoded by the ''MSN'' gene. Moesin (for membrane-organizing extension spike protein) is a member of the ERM protein family which includes ezrin and radixin. ERM proteins appear to function as cross-linkers between plasma membranes and actin-based cytoskeletons. Moesin is localized to filopodia and other membranous protrusions that are important for cell–cell recognition and signaling and for cell movement. Interactions Moesin has been shown to interact with: * CD43 * ICAM3 * Neutrophil cytosolic factor 1, * Neutrophil cytosolic factor 4 Neutrophil cytosol factor 4 is a protein that in humans is encoded by the ''NCF4'' gene. Function The protein encoded by this gene is a cytosolic regulatory component of the superoxide-producing phagocyte NADPH-oxidase, a multicomponent enzyme ... * VCAM-1 * EZR References Further reading * * * * * * * * * * * * * * * * {{NLM content Human proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ezrin
Ezrin also known as cytovillin or villin-2 is a protein that in humans is encoded by the ''EZR'' gene. Structure The N-terminus of ezrin contains a FERM domain which is further subdivided into three subdomains. The C-terminus contain an ERM domain. Function The cytoplasmic peripheral protein encoded by this gene can be phosphorylated by protein-tyrosine kinase in microvilli and is a member of the ERM protein family. This protein serves as a linker between plasma membrane and actin cytoskeleton. It plays a key role in cell surface structure adhesion, migration, and organization. The N-terminal domain (also called FERM domain) binds sodium-hydrogen exchanger regulatory factor ( NHERF) protein (involving long-range allostery). This binding can happen only when ezrin is in its active state. The activation of ezrin occurs in synergism of the two factors: 1) binding of the N-terminal domain to phosphatidylinositol(4,5)bis-phosphate ( PIP2) and 2) phosphorylation of threon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intercellular Adhesion Molecule
In molecular biology, intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecule-1 (VCAM-1) are part of the immunoglobulin superfamily. They are important in inflammation, immune responses and in intracellular signalling events. The ICAM family consists of five members, designated ICAM-1 to ICAM-5. They are known to bind to leucocyte integrins CD11/ CD18 such as LFA-1 and Macrophage-1 antigen, during inflammation and in immune responses. In addition, ICAMs may exist in soluble forms in human plasma, due to activation and proteolysis Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called protease ... mechanisms at cell surfaces. Mammalian intercellular adhesion molecules include: * ICAM-1 * ICAM2 * ICAM3 * ICAM4 * ICAM5 References Cell biology Protein families ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin Domain
The immunoglobulin domain, also known as the immunoglobulin fold, is a type of protein domain that consists of a 2-layer sandwich of 7-9 antiparallel β-strands arranged in two β-sheets with a Greek key topology, consisting of about 125 amino acids. The backbone switches repeatedly between the two β-sheets. Typically, the pattern is (N-terminal β-hairpin in sheet 1)-(β-hairpin in sheet 2)-(β-strand in sheet 1)-(C-terminal β-hairpin in sheet 2). The cross-overs between sheets form an "X", so that the N- and C-terminal hairpins are facing each other. Members of the immunoglobulin superfamily are found in hundreds of proteins of different functions. Examples include antibodies, the giant muscle kinase titin, and receptor tyrosine kinases. Immunoglobulin-like domains may be involved in protein–protein and protein–ligand interactions. Examples Human genes encoding proteins containing the immunoglobulin domain include: * A1BG * ACAM * ADAMTSL1 * ADAMTSL3 * AGE ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DC-SIGN
DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin) also known as CD209 ( Cluster of Differentiation 209) is a protein which in humans is encoded by the ''CD209'' gene. DC-SIGN is a C-type lectin receptor present on the surface of both macrophages and dendritic cells. DC-SIGN on macrophages recognises and binds with high affinity to high-mannose type N-glycans, a class of PAMPs (pathogen associated molecular patterns) commonly found on viruses, bacteria and fungi. This binding interaction activates phagocytosis. On myeloid and pre-plasmacytoid dendritic cells DC-SIGN mediates dendritic cell rolling interactions with blood endothelium and activation of CD4+ T cells, as well as recognition of pathogen haptens. Function DC-SIGN is a C-type lectin and has a high affinity for the ICAM3 molecule. It binds various microorganisms by recognizing high-mannose-containing glycoproteins on their surface, and can function as a co-receptor for several v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
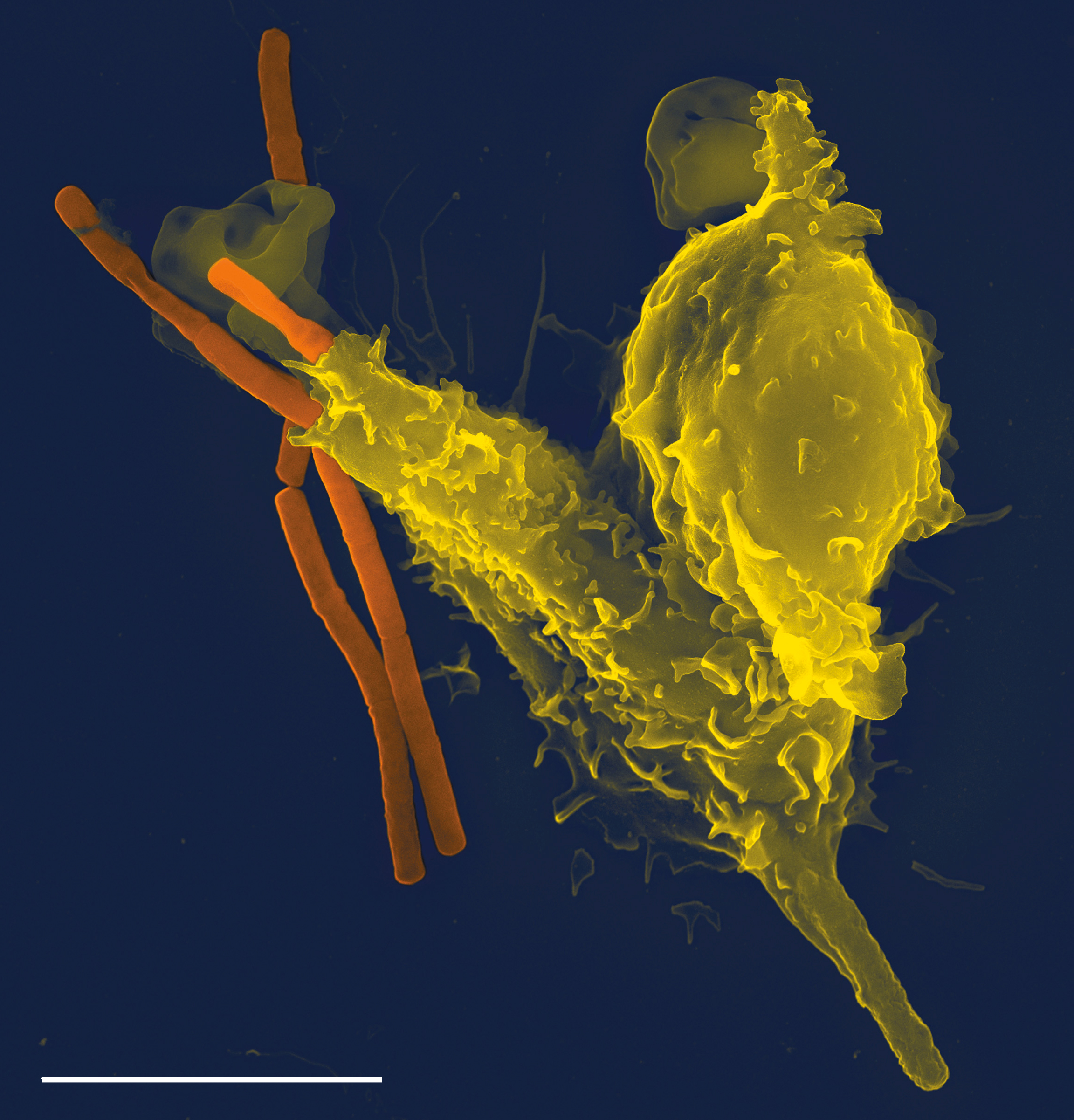
Phagocyte
Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek ', "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek ''kutos'', "hollow vessel". They are essential for fighting infections and for subsequent immunity. Phagocytes are important throughout the animal kingdom and are highly developed within vertebrates. One litre of human blood contains about six billion phagocytes. They were discovered in 1882 by Ilya Ilyich Mechnikov while he was studying starfish larvae.Ilya Mechnikov retrieved on November 28, 2008. Fro ''Physiology or Medicine 1901–1921 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ERM Protein Family
The ERM protein family consists of three closely related proteins, ezrin, radixin and moesin. The three paralogs, ezrin, radixin and moesin, are present in vertebrates, whereas other species have only one ERM gene. Therefore, in vertebrates these paralogs likely arose by gene duplication. ERM proteins are highly conserved throughout evolution. More than 75% identity is observed in the N-terminal and the C-terminal of vertebrates (ezrin, radixin, moesin), ''Drosophila'' (dmoesin) and ''C. elegans'' (ERM-1) homologs. Structure ERM molecules contain the following three domains: * N-terminal globular domain, also called FERM domain ( Band 4.1, ezrin, radixin, moesin). The FERM domain allows ERM proteins to interact with integral proteins of the plasma membrane, or scaffolding proteins localized beneath the plasma membrane. The FERM domain is composed of three subdomains (F1, F2, F3) that are arranged as a cloverleaf. * extended alpha-helical domain. * charged C-terminal domain. This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrin
Integrins are transmembrane receptors that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface (''e.g''. signal platelets to initiate an interaction with coagulation factors). Several types of integrins exist, and one cell generally has multiple different types on its surface. Integrins are found in all animals while integrin-like receptors are found in plant cells. Integrins work alongside other proteins such as cadherins, the immunoglobulin superfamily cell adhesion molecules, selectins and syndecans, to mediate cell–cell and cell–matrix interaction. Ligands for integrins include fibronectin, vitronectin, collagen and laminin. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lymphocyte Function-associated Antigen 1
Lymphocyte function-associated antigen 1 (LFA-1) is an integrin found on lymphocytes and other leukocytes. LFA-1 plays a key role in emigration, which is the process by which leukocytes leave the bloodstream to enter the tissues. LFA-1 also mediates firm arrest of leukocytes. Additionally, LFA-1 is involved in the process of cytotoxic T cell mediated killing as well as antibody mediated killing by granulocytes and monocytes. As of 2007, LFA-1 has 6 known ligands: ICAM-1, ICAM-2, ICAM-3, ICAM-4, ICAM-5, and JAM-A. LFA-1/ICAM-1 interactions have recently been shown to stimulate signaling pathways that influence T cell differentiation. LFA-1 belongs to the integrin superfamily of adhesion molecules. Structure LFA-1 is a heterodimeric glycoprotein with non-covalently linked subunits. LFA-1 has two subunits designated as the alpha subunit and beta subunit. The alpha subunit was named aL in 1983. The alpha subunit is designated CD11a; and the beta subunit, unique to leukocytes, is bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular Matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM. The animal extracellular matrix includes the interstitial matrix and the basement membrane. Interstitial matrix is present between various animal cells (i.e., in the intercellular spaces). Gels of polysaccharides and fibrous proteins fill the interstitial space and act as a compression buffer against the stress placed on the ECM. Basement membranes are sheet-like depositions of ECM on which various epithelial cells rest. Each type of conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |