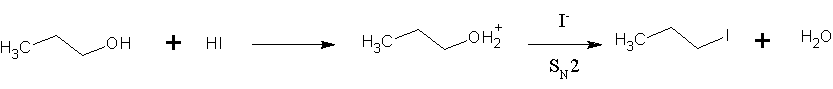|
Hexafluorothioacetone
Hexafluorothioacetone is an organic perfluoro thione compound with formula CF3CSCF3. At standard conditions it is a blue gas. Production Hexafluorothioacetone was first produced by Middleton in 1961 by boiling ''bis''-(perfluoroisopropyl)mercury with sulfur. Properties Hexafluorothioacetone boils at 8 °C. Below this it is a blue liquid. Colour The blue colour is due to absorption in the visible light range with bands at 800–675 nm and 725–400 nm. These bands are due to T1–S0 and S1–S0 transitions. There is also a strong absorption in ultraviolet around 230-190 nm. Reactions Hexafluorothioacetone acts more like a true thiocarbonyl (C=S) than many other thiocarbonyl compounds, because it is not able to form thioenol compounds (=C-S-H), and the sulfur is not in a negative ionized state (C-S−). Hexafluorothioacetone is not attacked by water or oxygen at standard conditions as are many other thiocarbonyls. Bases trigger the formation of a dimer 2,2,4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a musty odour. The most common form of this substance is hexafluoroacetone sesquihydrate (1.5 H2O), which is a hemihydrate of hexafluoropropane-2,2-diol , a geminal diol. Synthesis The industrial route to HFA involves treatment of hexachloroacetone with HF: :(CCl3)2CO + 6 HF → (CF3)2CO + 6 HCl Hexafluoropropylene oxide rearranges to give HFA. In the laboratory, HFA can be prepared in a two step process from perfluoropropene. In the first step KF catalyzes the reaction of the alkene with elemental sulfur to give the 1,3-dithietane CF3)2CSsub>2. This species is then oxidized by iodate to give (CF3)2CO. Uses Hexafluoroacetone is used in the production of hexafluoroisopropanol: :(CF3)2CO + H2 → (CF3)2CHOH It is also used as a precur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluoro
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound containing only carbon-fluorines and C−C bonds, as well as potentially heteroatoms. Perfluorinated compounds have properties that result from the presence of fluorocarbons (containing only C−F and C−C bonds) and any functional group. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method of production. Some of these compounds known as perfluoroalkanes can remain in our atmosphere for a long time. They bioaccumulate due to their chemical stability. Because of their potential contribution to climate change, they were regulated under the Kyoto Protocol. Some fluorosurfactants have proven toxic in animal testing while widespread industrial applications continue. Applications Perfluorinated compounds are used ubiquitously: For example, fluorosurfactants are widely used in the production of teflon (PTFE) and related fluorinated p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiolanone
A dithiolane is a sulfur heterocycle derived from cyclopentane by replacing two methylene bridges (-- units) with thioether groups. The parent compounds are 1,2-dithiolane and 1,3-dithiolane. 1,2-Dithiolanes are cyclic disulfides. Some dithiolanes are natural products that can be found in foods, such as asparagusic acid in asparagus. The 4-dimethylamino derivative nereistoxin was the inspiration for insecticides which act by blocking the nicotinic acetylcholine receptor. Lipoic acid is essential for aerobic metabolism in mammals and also has strong affinity with many metals including gold, molybdenum, and tungsten. Other 1,2-dithiolanes have relevance in nanomaterials such as gold nanoparticles or transition metal dichalcogenide monolayers (TMDs) ( MoS2 and WS2). Asparagusic-acid.png, asparagusic acid Nereistoxin.svg, nereistoxin, from which insecticides including cartap and bensultap were derived Lipoic acid.svg, lipoic acid 1,3-Dithiolanes are important as protecting grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorinated Compounds
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound containing only carbon-fluorines and C−C bonds, as well as potentially heteroatoms. Perfluorinated compounds have properties that result from the presence of fluorocarbons (containing only C−F and C−C bonds) and any functional group. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method of production. Some of these compounds known as perfluoroalkanes can remain in our atmosphere for a long time. They bioaccumulate due to their chemical stability. Because of their potential contribution to climate change, they were regulated under the Kyoto Protocol. Some fluorosurfactants have proven toxic in animal testing while widespread industrial applications continue. Applications Perfluorinated compounds are used ubiquitously: For example, fluorosurfactants are widely used in the production of teflon (PTFE) and related fluorinated poly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioketones
In organic chemistry, thioketones (; also known as thiones or thiocarbonyls) are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of , thioketones have the structure , which is reflected by the prefix "thio-" in the name of the functional group. Unhindered alkylthioketones typically tend to form polymers or rings. Structure and bonding The C=S bond length of thiobenzophenone is 1.63 Å, which is comparable to 1.64 Å, the C=S bond length of thioformaldehyde, measured in the gas phase. Due to steric interactions, the phenyl groups are not coplanar and the dihedral angle SC-CC is 36°. Unhindered dialkylthiones polymerize or oligomerize but thiocamphor is well characterized red solid. Consistent with the double bond rule, most alkyl thioketones are unstable with respect to dimerization.Organosulfur Chemistry I: Topics in Current Chemistry, 1999, Volume 204/1999, 127-181, The energy difference between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition. Classes Dienes can be divided into three classes, depending on the relative location of the double bonds: #Cumulated dienes have the double bonds sharing a common atom. The result is more specifically called an allene. #Conjugated dienes have conjugated double bonds separated by one single bond. Conjugated dienes are more stable than other dienes because of resonance. #Unconjugated dienes have the double bonds separated by two or more single bonds. They are usually less stable t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazomethane
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane. Use For safety and convenience diazomethane is always prepared as needed as a solution in ether and used as such. It converts carboxylic acids to methyl esters and phenols into their methyl ethers. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give methyldiazonium cation, which reacts with the carboxylate ion to give the methyl ester and nitrogen gas. L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Iodide
Hydrogen iodide () is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent. Properties of hydrogen iodide HI is a colorless gas that reacts with oxygen to give water and iodine. With moist air, HI gives a mist (or fumes) of hydroiodic acid. It is exceptionally soluble in water, giving hydroiodic acid. One liter of water will dissolve 425 liters of HI gas, the most concentrated solution having only four water molecules per molecule of HI. Hydroiodic acid Hydroiodic acid is not pure hydrogen iodide, but a mixture containing it. Commercial "concentrated" hydroiodic acid usually contains 48–57% HI by ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C. Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached. Hydrogen bromide, and its aqueous solution, are commonly used reagents in the preparation of bromide compounds. Reactions Organic chemistry Hydrogen bromide and hydrobromic acid are important reagents in the production of organobromine compounds.Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements; Butterworth-Heineman: Oxford, Great Britain; 1997; pp. 809–812.Vollhardt, K. P. C.; Schore, N. E. Organic Chemistry: Structure and Function; 4th Ed.; W. H. Freeman and Company: New York, NY; 2003. In a free- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |