|
Hsc70 Heat-shock Proteins
Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the ''HSPA8'' gene on chromosome 11. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including signal transduction, apoptosis, autophagy, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence, and aging. Structure This gene encodes a 70kDa heat shock protein which is a member of the heat shock protein 70 (Hsp70) family. As a Hsp70 protein, it has a C-terminal protein substrate-binding domain and an N-terminal ATP-binding domain. The substrate-binding domain consists of two subdomains, a two-layered β-sandwich subdomain (SBDβ) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Shock Protein
Heat shock proteins (HSP) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including exposure to cold, UV light and during wound healing or tissue remodeling. Many members of this group perform chaperone functions by stabilizing new proteins to ensure correct folding or by helping to refold proteins that were damaged by the cell stress. This increase in expression is transcriptionally regulated. The dramatic upregulation of the heat shock proteins is a key part of the heat shock response and is induced primarily by heat shock factor (HSF). HSPs are found in virtually all living organisms, from bacteria to humans. Heat-shock proteins are named according to their molecular weight. For example, HSP60, Hsp60, Hsp70 and Hsp90 (the most widely studied HSPs) refer to families of heat shock proteins on the order of 60, 70 and 90 ato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
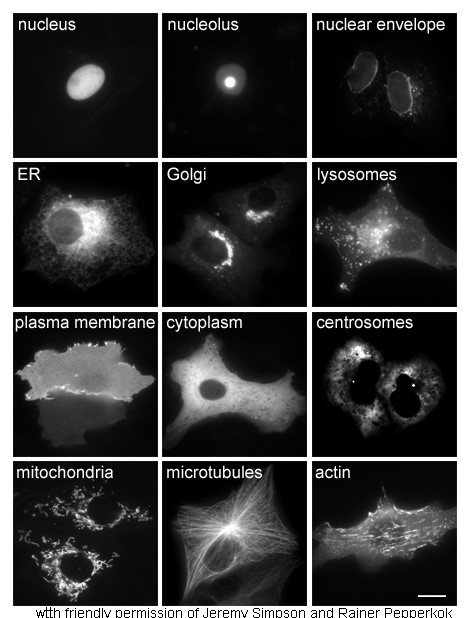
Cytoplasm
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are cytosol (a gel-like substance), the organelles (the cell's internal sub-structures), and various cytoplasmic inclusions. The cytoplasm is about 80% water and is usually colorless. The submicroscopic ground cell substance or cytoplasmic matrix which remains after exclusion of the cell organelles and particles is groundplasm. It is the hyaloplasm of light microscopy, a highly complex, polyphasic system in which all resolvable cytoplasmic elements are suspended, including the larger organelles such as the ribosomes, mitochondria, the plant plastids, lipid droplets, and vacuoles. Most cellular activities take place within the cytoplasm, such as many metabolic pathways including glycolysis, and proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Nucleus
The cell nucleus (pl. nuclei; from Latin or , meaning ''kernel'' or ''seed'') is a membrane-bound organelle found in eukaryotic cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, have no nuclei, and a few others including osteoclasts have many. The main structures making up the nucleus are the nuclear envelope, a double membrane that encloses the entire organelle and isolates its contents from the cellular cytoplasm; and the nuclear matrix, a network within the nucleus that adds mechanical support. The cell nucleus contains nearly all of the cell's genome. Nuclear DNA is often organized into multiple chromosomes – long stands of DNA dotted with various proteins, such as histones, that protect and organize the DNA. The genes within these chromosomes are structured in such a way to promote cell function. The nucleus maintains the integrity of genes and controls the activities of the cell by regulating gene expres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strokes
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functioning properly. Signs and symptoms of a stroke may include an inability to move or feel on one side of the body, problems understanding or speaking, dizziness, or loss of vision to one side. Signs and symptoms often appear soon after the stroke has occurred. If symptoms last less than one or two hours, the stroke is a transient ischemic attack (TIA), also called a mini-stroke. A hemorrhagic stroke may also be associated with a severe headache. The symptoms of a stroke can be permanent. Long-term complications may include pneumonia and loss of bladder control. The main risk factor for stroke is high blood pressure. Other risk factors include high blood cholesterol, tobacco smoking, obesity, diabetes mellitus, a previous TIA, end-stag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heart Attacks
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may travel into the shoulder, arm, back, neck or jaw. Often it occurs in the center or left side of the chest and lasts for more than a few minutes. The discomfort may occasionally feel like heartburn. Other symptoms may include shortness of breath, nausea, feeling faint, a cold sweat or feeling tired. About 30% of people have atypical symptoms. Women more often present without chest pain and instead have neck pain, arm pain or feel tired. Among those over 75 years old, about 5% have had an MI with little or no history of symptoms. An MI may cause heart failure, an irregular heartbeat, cardiogenic shock or cardiac arrest. Most MIs occur due to coronary artery disease. Risk factors include high blood pressure, smoking, diabetes, lack of exercis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Embryologic
Embryology (from Greek ἔμβρυον, ''embryon'', "the unborn, embryo"; and -λογία, ''-logia'') is the branch of animal biology that studies the prenatal development of gametes (sex cells), fertilization, and development of embryos and fetuses. Additionally, embryology encompasses the study of congenital disorders that occur before birth, known as teratology. Early embryology was proposed by Marcello Malpighi, and known as preformationism, the theory that organisms develop from pre-existing miniature versions of themselves. Aristotle proposed the theory that is now accepted, epigenesis. Epigenesis is the idea that organisms develop from seed or egg in a sequence of steps. Modern embryology, developed from the work of Karl Ernst von Baer, though accurate observations had been made in Italy by anatomists such as Aldrovandi and Leonardo da Vinci in the Renaissance. Comparative embryology Preformationism and epigenesis As recently as the 18th century, the prevai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquitylation
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A. The addition of ubiquitin to a substrate protein is called ubiquitylation (or, alternatively, ubiquitination or ubiquitinylation). Ubiquitylation affects proteins in many ways: it can mark them for degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitylation involves three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade is to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond, cyst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone-mediated Autophagy
Chaperone-mediated autophagy (CMA) refers to the chaperone-dependent selection of soluble cytosolic proteins that are then targeted to lysosomes and directly translocated across the lysosome membrane for degradation. The unique features of this type of autophagy are the selectivity on the proteins that are degraded by this pathway and the direct shuttling of these proteins across the lysosomal membrane without the requirement for the formation of additional vesicles (Figure 1). Molecular components and steps The proteins that are degraded through CMA are cytosolic proteins or proteins from other compartments once they reach the cytosol. Therefore, some of the components that participate in CMA are present in the cytosol while others are located at the lysosomal membrane (Table I). Specific selection of proteins for degradation in all forms of autophagy came to further understanding as studies discovered the role of chaperones like hsc70. Although hsc70 targets cytosolic protein to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synaptojanin
Synaptojanin is a protein involved in vesicle uncoating in neurons. This is an important regulatory lipid phosphatase. It dephosphorylates the D-5 position phosphate from phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and Phosphatidylinositol (4,5)-bisphosphate(PIP2). It belongs to family of 5-phosphatases, which are structurally unrelated to D-3 inositol phosphatases like PTEN. Other members of the family of 5'phosphoinositide phosphatases include OCRL, SHIP1, SHIP2, INPP5J, INPP5E, INPP5B, INPP5A and SKIP. Synaptojanin Family The synaptojanin family comprises proteins that are key players in the synaptic vesicle recovery at the synapse. In general, vesicles containing neurotransmitters fuse with the presynaptic cell in order to release neurotransmitter into the synaptic cleft. It is the release of neurotransmitters that allows neuron to neuron communication in the nervous system. The recovery of the vesicle is referred to as endocytosis and is important to reset the pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNAJC6
Putative tyrosine-protein phosphatase auxilin is an enzyme that in humans is encoded by the ''DNAJC6'' gene. Function DNAJC6 belongs to the evolutionarily conserved DNAJ/HSP40 family of proteins, which regulate molecular chaperone activity by stimulating ATPase activity. DNAJ proteins may have up to 3 distinct domains: a conserved 70-amino acid J domain, usually at the N terminus, a glycine/phenylalanine (G/F)-rich region, and a cysteine-rich domain containing 4 motifs resembling a zinc-finger domain (Ohtsuka and Hata, 2000). Structure The protein tyrosine phosphatase domain and C2 domain pair of auxilin, located near the N-terminus of the polypeptide, constitute a superdomain, a tandem arrangement of two or more nominally unrelated domains that form a single heritable unit. The phosphatase domain belongs to the auxilin subfamily Putative tyrosine-protein phosphatase auxilin is an enzyme that in humans is encoded by the ''DNAJC6'' gene. Function DNAJC6 belongs to the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clathrin
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle, hence the protein's name, which is derived from the Latin ''clathrum'' meaning lattice. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection. Structure The clathrin triskelion is composed of three clathrin heavy chains inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATPase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, SV40 T-antigen, ATP hydrolase, complex V (mitochondrial electron transport), (Ca2+ + Mg2+)-ATPase, HCO3−-ATPase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life. Some such enzymes are integral membrane proteins (anchored within biological membranes), and move solutes across the membrane, typically against their concentration gradient. These are called transmembrane ATPases. Functions Transmembrane ATPases import metabolites necessary for cell metabolism and export toxins, wastes, and solutes that can hinder cellular processes. An important example is the sodium-potass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |