|
Hot Water Storage
A hot water storage tank (also called a hot water tank, thermal storage tank, hot water thermal storage unit, heat storage tank and hot water cylinder) is a water tank used for storing hot water for space heating or domestic use. Water is a convenient heat storage medium because it has a high specific heat capacity. This means, compared to other substances, it can store more heat per unit of weight. Water is non-toxic and low cost. An efficiently insulated tank can retain stored heat for days, reducing fuel costs. Hot water tanks may have a built-in gas or oil burner system, electric immersion heaters. Some types use an external heat exchanger such as a central heating system, or heated water from another energy source. The most typical, in the domestic context, is a fossil-fuel burner, electric immersion elements, or a district heating scheme. Water heaters for washing, bathing, or laundry have thermostat controls to regulate the temperature, in the range of , and are connect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Thermal Collector
A solar thermal collector collects heat by absorbing sunlight. The term "solar collector" commonly refers to a device for solar hot water heating, but may refer to large power generating installations such as solar parabolic troughs and solar towers or non water heating devices such as solar air heaters. Solar thermal collectors are either non-concentrating or concentrating. In non-concentrating collectors, the aperture area (i.e., the area that receives the solar radiation) is roughly the same as the absorber area (i.e., the area absorbing the radiation). A common example of such a system is a metal plate that is painted a dark color to maximize the absorption of sunlight. The energy is then collected by cooling the plate with a working fluid, often water or glycol running in pipes attached to the plate. Concentrating collectors have a much larger aperture than the absorber area. The aperture is typically in the form of a mirror that is focussed on the absorber, which in mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Conductor
Conduction is the process by which heat is transferred from the hotter end to the colder end of an object. The ability of the object to conduct heat is known as its ''thermal conductivity'', and is denoted . Heat spontaneously flows along a temperature gradient (i.e. from a hotter body to a colder body). For example, heat is conducted from the hotplate of an electric stove to the bottom of a saucepan in contact with it. In the absence of an opposing external driving energy source, within a body or between bodies, temperature differences decay over time, and thermal equilibrium is approached, temperature becoming more uniform. In conduction, the heat flow is within and through the body itself. In contrast, in heat transfer by thermal radiation, the transfer is often between bodies, which may be separated spatially. Heat can also be transferred by a combination of conduction and radiation. In solids, conduction is mediated by the combination of vibrations and collisions of molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Heating System
A solar thermal collector collects heat by Absorption (optics), absorbing sunlight. The term "solar collector" commonly refers to a device for solar hot water panel, solar hot water heating, but may refer to large power generating installations such as solar parabolic troughs and Solar power tower, solar towers or non water heating devices such as solar air heaters. Solar thermal collectors are either non-concentrating or concentrating. In non-concentrating collectors, the aperture area (i.e., the area that receives the solar radiation) is roughly the same as the absorber area (i.e., the area absorbing the radiation). A common example of such a system is a metal plate that is painted a dark color to maximize the absorption of sunlight. The energy is then collected by cooling the plate with a working fluid, often water or glycol running in pipes attached to the plate. Concentrating collectors have a much larger aperture than the absorber area. The aperture is typically in the fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Control System
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stratification (water)
Stratification is the separation of water in layers. Two main types of stratification of water are uniform and layered stratification. Layered stratification occurs in all ocean basins. Stratified layers act as a barrier to the mixing of water, which impacts the exchange of heat, carbon, oxygen and other nutrients. Due to upwelling and downwelling, which are both wind-driven, mixing of different layers can occur through the rise of cold nutrient-rich and warm water, respectively. Generally, layers are based on water density: heavier, and hence denser, water is below the lighter water, representing a stable stratification. For example, the pycnocline is a layer in the ocean where the change in density is relatively large compared to that of other layers in the ocean. The thickness of the thermocline is not constant everywhere and depends on a variety of variables. Between 1960 and 2018, upper ocean stratification increased between 0.7-1.2% per decade. This means that the differenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermocline
A thermocline (also known as the thermal layer or the metalimnion in lakes) is a thin but distinct layer in a large body of fluid (e.g. water, as in an ocean or lake; or air, e.g. an atmosphere) in which temperature changes more drastically with depth than it does in the layers above or below. In the ocean, the thermocline divides the upper mixed layer from the calm deep water below. Depending largely on season, latitude, and turbulent mixing by wind, thermoclines may be a semi-permanent feature of the body of water in which they occur, or they may form temporarily in response to phenomena such as the radiative heating/cooling of surface water during the day/night. Factors that affect the depth and thickness of a thermocline include seasonal weather variations, latitude, and local environmental conditions, such as tides and currents. Oceans Most of the heat energy of the sunlight that strikes the Earth is absorbed in the first few centimeters at the ocean's surface, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Descaling Agent
A descaling agent or chemical descaler is a liquid chemical substance used to remove limescale from metal surfaces in contact with hot water, such as in boilers, water heaters, and kettles. Limescale is either white or brown in colour due to the presence of iron compounds. Glass surfaces may also exhibit scaling stains, as can many ceramic surfaces present in bathrooms and kitchen, and descaling agents can be used safely to remove those stains without affecting the substrate since both ceramics and glass are unreactive to most acids. Action Descaling agents are typically acidic compounds such as hydrochloric acid that react with the calcium carbonate and magnesium carbonate compounds present in the scale, producing carbon dioxide gas and a soluble salt. CaCO3(s) + 2H+(aq) → Ca2+(aq) + CO2(g) + H2O(l) MgCO3(s) + 2H+(aq) → Mg2+(aq) + CO2(g) + H2O(l) Strongly acidic descaling agents are usually corrosive to the eyes and skin, and can also attack and degrade clothing f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
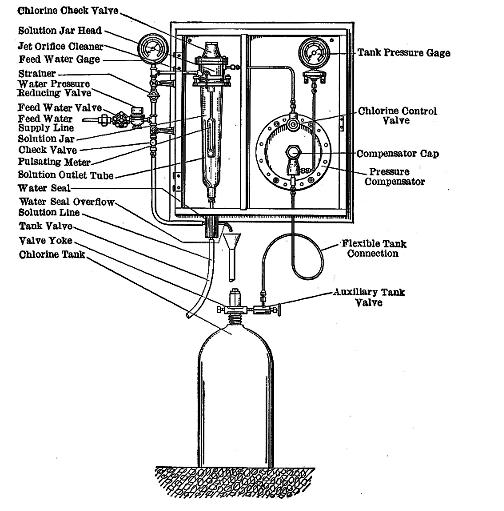
Water Chlorination
Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid. History In a paper published in 1894, it was formally proposed to add chlorine to water to render it "germ-free". Two other authorities endorsed this proposal and published it in many other papers in 1895. Early attempts at implementing water chlorination at a water treatment plant were made in 1893 in Hamburg, Germany. In 1897 the town of Maidstone, England was the first to have its entire water supply treated with chlorine. Permanent water chlorination began in 1905, when a faulty slow sand filter and a contaminated water supply caused a serious typhoid fever epidemic in Lincoln, England. Alexander Cruickshank Houston used chlorination of the water to stop the epidemic. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sacrificial Anode
A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged metal structures from corrosion. They are made from a metal alloy with a more "active" voltage (more negative reduction potential / more positive electrode potential) than the metal of the structure. The difference in potential between the two metals means that the galvanic anode corrodes, in effect being "sacrificed" in order to protect the structure. Theory In brief, corrosion is a chemical reaction occurring by an electrochemical mechanism (a redox reaction).Shrier 10:4 During corrosion of iron or steel there are two reactions, oxidation (equation ), where electrons leave the metal (and the metal dissolves, i.e. actual loss of metal results) and reduction, where the electrons are used to convert oxygen and water to hydroxide ions (equation ): In most environments, the hydroxide ions and ferrous ions combine to form ferrous hydroxide, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stainless Steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corrosion resistance, resistance to corrosion results from the chromium, which forms a Passivation (chemistry), passive film that can protect the material and self-healing material, self-heal in the presence of oxygen. The alloy's properties, such as luster and resistance to corrosion, are useful in many applications. Stainless steel can be rolled into Sheet metal, sheets, plates, bars, wire, and tubing. These can be used in cookware, cutlery, surgical instruments, major appliances, vehicles, construction material in large buildings, industrial equipment (e.g., in paper mills, chemical plants, water treatment), and storage tanks and tankers for chemicals and food products. The biological cleanability of stainless steel is superior to both alumi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |