|
Hexadecane
Hexadecane (also called cetane) is an alkane hydrocarbon with the chemical formula C16H34. Hexadecane consists of a chain of 16 carbon atoms, with three hydrogen atoms bonded to the two end carbon atoms, and two hydrogens bonded to each of the 14 other carbon atoms. Cetane number ''Cetane'' is often used as a shorthand for cetane number, a measure of the combustion of diesel fuel. Cetane ignites very easily under compression; for this reason, it is assigned a cetane number of 100, and serves as a reference for other fuel mixtures. Hexadecyl radical Hexadecyl is an alkyl radical of carbon and hydrogen derived from hexadecane, with formula C16H33 and with mass 225.433, occurring especially in cetyl alcohol. It confers strong hydrophobicity on molecules containing it. Carboplatin modified with hexadecyl and polyethylene glycol has increased liposolubility and PEGylation, proposed to useful in chemotherapy, specifically non-small-cell lung cancer. Hexadecyl was used from 1982 for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentadecane
Pentadecane is an alkane hydrocarbon with the chemical formula C15H32. It can be monoterminally oxidized Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ... to 1-pentadecanol. References Alkanes {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptadecane
Heptadecane is an organic compound, an alkane hydrocarbon with the chemical formula C17H36. The name may refer to any of 24894 theoretically possible structural isomers, or to a mixture thereof. The unbranched isomer is normal or ''n''-heptadecane, CH3(CH2)15CH3. In the IUPAC nomenclature, the name of this compound is simply heptadecane, since the other isomers are viewed and named as alkyl-substituted versions of smaller alkanes. The most compact and branched isomer would be tetra-''tert''-butylmethane, but its existence is believed to be impossible due to steric hindrance Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions .... Indeed, it is believed to be the smallest "impossible" alkane. References External links List of plant species containing heptadecane Dr. Duke's Phytochem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like pentacontane () or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula , and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote ''any'' saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes) or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Higher Alkanes
Higher alkanes are alkanes having nine or more carbon atoms. Nonane is the lightest alkane to have a flash point above 25 °C, and is not classified as dangerously flammable. The term ''higher alkanes'' is sometimes used literally as "alkanes with a higher number of carbon atoms". One definition distinguishes the higher alkanes as the n-alkanes that are solid under natural conditions. Uses Alkanes from nonane to hexadecane (those alkanes with nine to sixteen carbon atoms) are liquids of higher viscosity, which are less suitable for use in gasoline. They form instead the major part of diesel and aviation fuel. Diesel fuels are characterised by their cetane number, cetane being an older name for hexadecane. However the higher melting points of these alkanes can cause problems at low temperatures and in polar regions, where the fuel becomes too thick to flow correctly. Mixtures of the normal alkanes are used as boiling point standards for simulated distillation by gas chroma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Handbook Of Chemistry And Physics
The ''CRC Handbook of Chemistry and Physics'' is a comprehensive one-volume reference resource for science research. First published in 1914, it is currently () in its 103rd edition, published in 2022. It is sometimes nicknamed the "Rubber Bible" or the "Rubber Book", as CRC originally stood for "Chemical Rubber Company". As late as the 1962–1963 edition (3604 pages) the ''Handbook'' contained myriad information for every branch of science and engineering. Sections in that edition include: Mathematics, Properties and Physical Constants, Chemical Tables, Properties of Matter, Heat, Hygrometric and Barometric Tables, Sound, Quantities and Units, and Miscellaneous. Earlier editions included sections such as "Antidotes of Poisons", "Rules for Naming Organic Compounds", "Surface Tension of Fused Salts", "Percent Composition of Anti-Freeze Solutions", "Spark-gap Voltages", "Greek Alphabet", "Musical Scales", "Pigments and Dyes", "Comparison of Tons and Pounds", "Twist Drill and St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocetane
Isocetane (2,2,4,4,6,8,8-heptamethylnonane) is a highly branched alkane used as a reference in determining the cetane number of diesel. It has a cetane number of 15. Isocetane replaced 1-methylnaphthalene in 1962 as the lower reference for cetane number (1-methylnaphthalene has cetane number zero) owing to the oxidation instability and difficulty of use of 1-methylnaphthalene in the reference engine. Strictly speaking, if the standard meaning of ‘iso’ is followed, the name ''isocetane'' should be reserved for the isomer 2-methylpentadecane. However, 2,2,4,4,6,8,8-heptamethylnonane is by far the most important isomer of cetane Hexadecane (also called cetane) is an alkane hydrocarbon with the chemical formula C16H34. Hexadecane consists of a chain of 16 carbon atoms, with three hydrogen atoms bonded to the two end carbon atoms, and two hydrogens bonded to each of the 14 ... and so, historically, it has ended up with this name. References {{Organic-compound-stub Alkanes [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cetane Index
Cetane index is used as a substitute for the cetane number of diesel fuel. The cetane index is calculated based on the fuel's density and distillation range ( ASTM D86). There are two methods used, ASTM D976 and D4737. The older D976, or "two-variable equation" is outdated and should no longer be used for cetane number estimation. It is, however, still required by the United States Environmental Protection Agency (EPA) as an alternative method for satisfying its aromaticity requirement for diesel fuel. D4737 is the newest method and is sometimes referred to as "the four-variable equation". D4737 is the same method as ISO 4264. Cetane index in some crude oil assays is often referred to as Cetane calcule, while the cetane number is referred to as Cetane measure. Cetane improver additives Cetane index calculations can not account for cetane improver additives and therefore do not measure total cetane number for additized diesel fuels. Diesel engine operation is primarily related ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasmalogens
Glycerophospholipids of biochemical relevance are divided into three subclasses based on the substitution present at the sn-1 position of the glycerol backbone: acyl, alkyl and alkenyl. Of these, the alkyl and alkenyl moiety in each case form an ether bond, which makes for two types of ether phospholipids, plasmanyl (alkyl moiety at sn-1), and plasmenyl (alkenyl moiety with vinyl ether linkage at sn-1). Plasmalogens are plasmenyls with an ester (acyl group) linked lipid at the sn-2 position of the glycerol backbone, chemically designated 1-0(1Z-alkenyl)-2-acyl-glycerophospholipids. The lipid attached to the vinyl ether at sn-1 can be C16:0, C18:0, or C18:1 (saturated and monounsaturated), and the lipid attached to the acyl group at sn-2 can be C22:6 ω-3 (docosahexaenoic acid) or C20:4 ω-6 (arachidonic acid), (both are polyunsaturated acids). Plasmalogens are classified according to their head group, mainly as PC plasmalogens (plasmenylcholines) and PE plasmalogens (plasmenylethal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism. The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g.NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
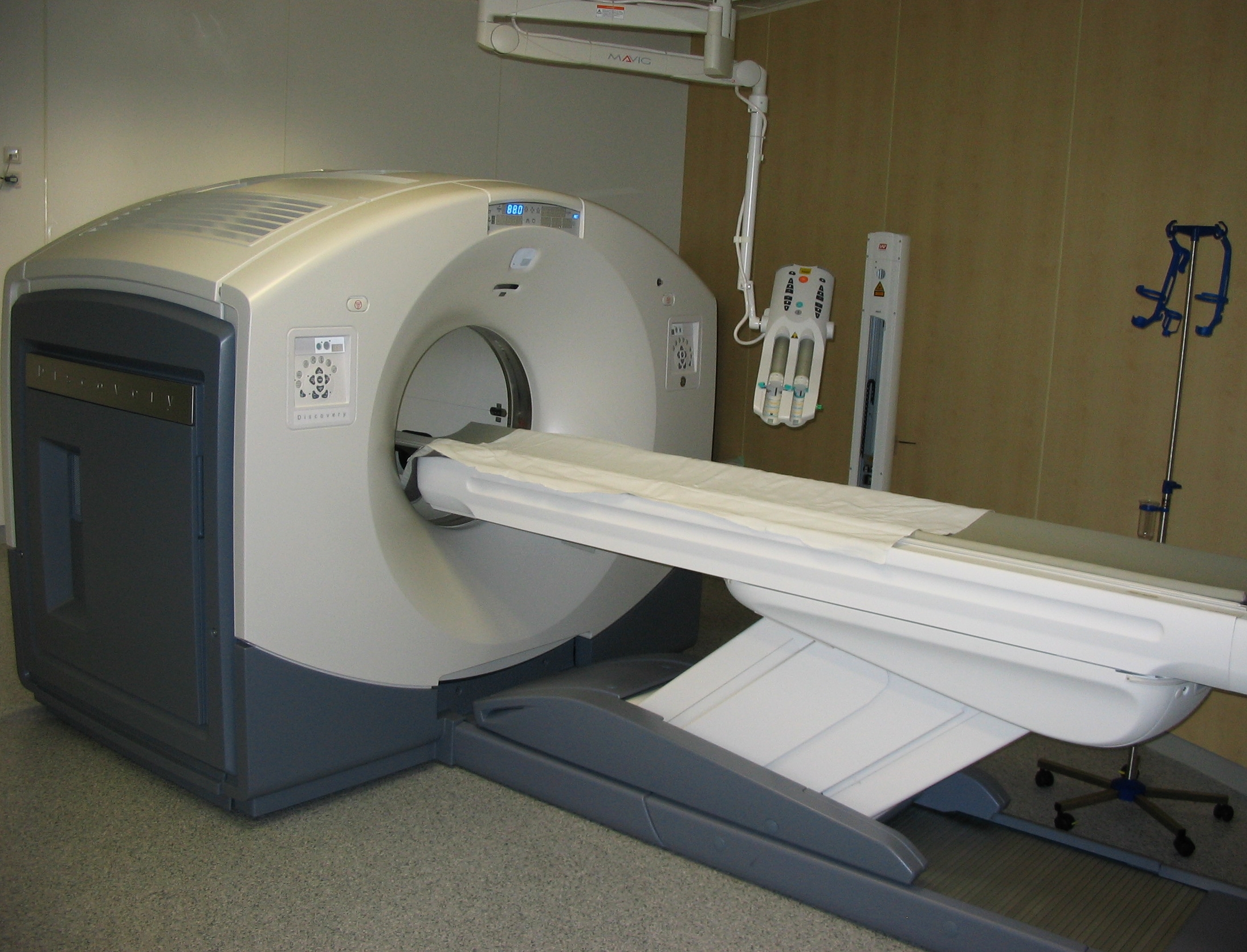
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in Metabolism, metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example, 18F-FDG, -FDG is commonly used to detect cancer, Sodium fluoride#Medical imaging, NaF is widely used for detecting bone formation, and Isotopes of oxygen#Oxygen-15, oxygen-15 is sometimes used to measure blood flow. PET is a common medical imaging, imaging technique, a Scintigraphy#Process, medical scintillography technique used in nuclear medicine. A radiopharmaceutical, radiopharmaceutical — a radioisotope attached to a drug — is injected into the body as a radioactive tracer, tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogels
A gel is a semi-solid that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state, although the liquid phase may still diffuse through this system. A gel has been defined phenomenologically as a soft, solid or solid-like material consisting of two or more components, one of which is a liquid, present in substantial quantity. By weight, gels are mostly liquid, yet they behave like solids because of a three-dimensional cross-linked network within the liquid. It is the crosslinking within the fluid that gives a gel its structure (hardness) and contributes to the adhesive stick (tack). In this way, gels are a dispersion of molecules of a liquid within a solid medium. The word ''gel'' was coined by 19th-century Scottish chemist Thomas Graham by clipping from ''gelatine''. The process of forming a gel is called gelation. IUPAC definition } Com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |