|
Heat Capacity Rate
The heat capacity rate is heat transfer terminology used in thermodynamics and different forms of engineering denoting the quantity of heat a flowing fluid of a certain mass flow rate is able to absorb or release per unit temperature change per unit time. It is typically denoted as ''C'', listed from empirical data experimentally determined in various reference works, and is typically stated as a comparison between a hot and a cold fluid, ''C''h and ''C''c either graphically, or as a linearized equation. It is an important quantity in heat exchanger technology common to either heating or cooling systems and needs, and the solution of many real world problems such as the design of disparate items as different as a microprocessor and an internal combustion engine. Basis A hot fluid's heat capacity rate can be much greater than, equal to, or much less than the heat capacity rate of the same fluid when cold. In practice, it is most important in specifying heat-exchanger systems, where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its thermal mass. Definition Basic definition The heat capacity of an object, denoted by C, is the limit : C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably depending on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Transfer
Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, Convection (heat transfer), thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species (mass transfer in the form of advection), either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system. Heat conduction, also called diffusion, is the direct microscopic exchanges of kinetic energy of particles (such as molecules) or quasiparticles (such as lattice waves) through the boundary between two systems. When an object is at a different temperature from another body or its surroundings, heat flows so that the body and the surroundings reach the same temperature, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volumetric Heat Capacity
The volumetric heat capacity of a material is the heat capacity of a sample of the substance divided by the volume of the sample. It is the amount of energy that must be added, in the form of heat, to one unit of volume of the material in order to cause an increase of one unit in its temperature. The SI unit of volumetric heat capacity is joule per kelvin per cubic meter, J⋅K−1⋅m−3. The volumetric heat capacity can also be expressed as the specific heat capacity (heat capacity per unit of mass, in J⋅K−1⋅ kg−1) times the density of the substance (in kg/ L, or g/ mL). This quantity may be convenient for materials that are commonly measured by volume rather than mass, as is often the case in engineering and other technical disciplines. The volumetric heat capacity often varies with temperature, and is different for each state of matter. While the substance is undergoing a phase transition, such as melting or boiling, its volumetric heat capacity is technically in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Equations
Thermodynamics is expressed by a mathematical framework of ''thermodynamic equations'' which relate various thermodynamic quantities and physical properties measured in a laboratory or production process. Thermodynamics is based on a fundamental set of postulates, that became the laws of thermodynamics. Introduction One of the fundamental thermodynamic equations is the description of thermodynamic work in analogy to mechanical work, or weight lifted through an elevation against gravity, as defined in 1824 by French physicist Sadi Carnot. Carnot used the phrase motive power for work. In the footnotes to his famous ''On the Motive Power of Fire'', he states: “We use here the expression ''motive power'' to express the useful effect that a motor is capable of producing. This effect can always be likened to the elevation of a weight to a certain height. It has, as we know, as a measure, the product of the weight multiplied by the height to which it is raised.” With the inc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics. Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic work and heat transfer as defined in thermodynamics, but the kelvin was redefined by international agreement in 2019 in terms of phenomena that are now understood as manifestations of the kinetic energy of free motion of microscopic particles such as atoms, molecules, and electrons. From the thermodynamic viewpoint, for historical reasons, because of how it is defined and measured, this microscopic kinetic definition is regarded as an "empirical" temperature. It was adopted because in practice it can generally be measured more precisely than can Kelvin's thermodynamic temperature. A thermodynamic temperature reading of zero is of particular importance for the third law of thermodynamics. By convention, it is reported on the ''Kelvin scale'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), the latter being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible to extract energy as heat from a body at that temperature. Temperature is important in all fields of natur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Melting Heat
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a solid to a liquid, at constant pressure. It is the amount of energy required to convert one mole of solid into liquid For example, when melting 1 kg of ice (at 0 °C under a wide range of pressures), 333.55 kJ of energy is absorbed with no temperature change. The heat of solidification (when a substance changes from liquid to solid) is equal and opposite. This energy includes the contribution required to make room for any associated change in volume by displacing its environment against ambient pressure. The temperature at which the phase transition occurs is the melting point or the freezing point, according to context. By convention, the pressure is assumed to be unless otherwise specified. Overview The 'enthalpy' of fusi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
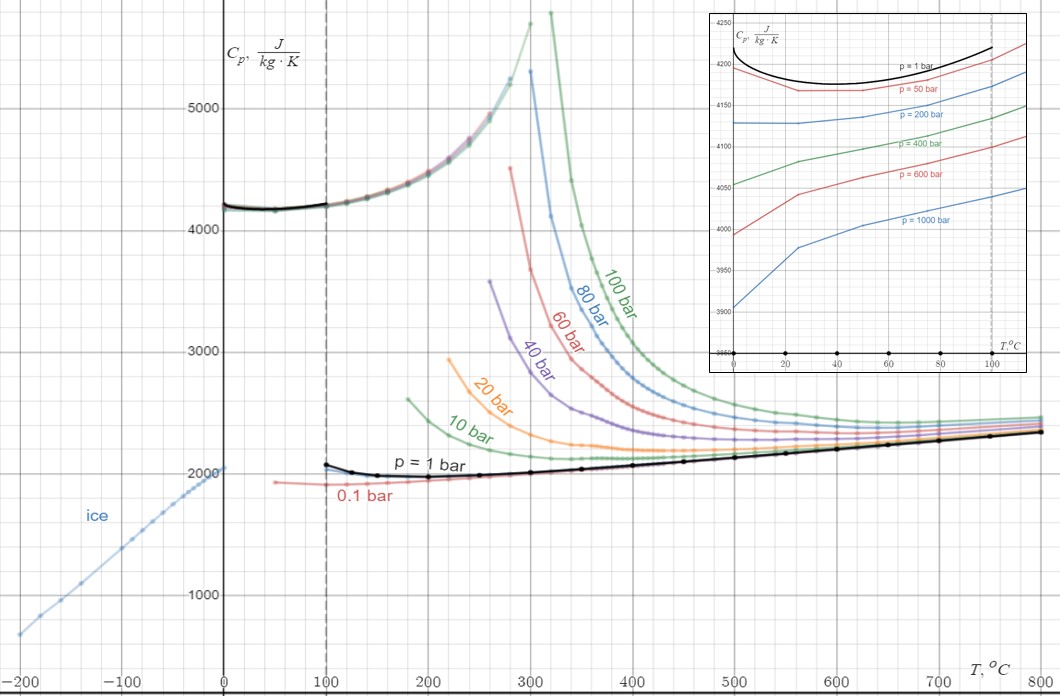
Specific Heat Capacity
In thermodynamics, the specific heat capacity (symbol ) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. The SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of of water by is , so the specific heat capacity of water is . Specific heat capacity often varies with temperature, and is different for each state of matter. Liquid water has one of the highest specific heat capacities among common substances, about at 20 °C; but that of ice, just below 0 °C, is only . The specific heat capacities of iron, granite, and hydrogen gas are about 449 J⋅kg−1⋅K−1, 790 J⋅kg−1⋅K−1, and 14300 J⋅kg−1⋅K−1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latent Heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process — usually a first-order phase transition. Latent heat can be understood as energy in hidden form which is supplied or extracted to change the state of a substance without changing its temperature. Examples are latent heat of fusion and latent heat of vaporization involved in phase changes, i.e. a substance condensing or vaporizing at a specified temperature and pressure. The term was introduced around 1762 by Scottish chemist Joseph Black. It is derived from the Latin ''latere'' (''to lie hidden''). Black used the term in the context of calorimetry where a heat transfer caused a volume change in a body while its temperature was constant. In contrast to latent heat, sensible heat is energy transferred as heat, with a resultant temperature change in a body. Usage The terms ″sensible heat″ and ″laten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Transfer Coefficient
In thermodynamics, the heat transfer coefficient or film coefficient, or film effectiveness, is the proportionality constant between the heat flux and the thermodynamic driving force for the flow of heat (i.e., the temperature difference, ). It is used in calculating the heat transfer, typically by convection or phase transition between a fluid and a solid. The heat transfer coefficient has SI units in watts per square meter kelvin (W/m2/K). The overall heat transfer rate for combined modes is usually expressed in terms of an overall conductance or heat transfer coefficient, . In that case, the heat transfer rate is: :\dot=hA(T_2-T_1) where (in SI units): *: surface area where the heat transfer takes place (m2) *: temperature of the surrounding fluid (K) *: temperature of the solid surface (K) The general definition of the heat transfer coefficient is: :h = \frac where: *: heat flux (W/m2); i.e., thermal power per unit area, q = d\dot/dA *: difference in temperature bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |