|
Gas Laser
A gas laser is a laser in which an electric current is discharged through a gas to produce coherent light. The gas laser was the first continuous-light laser and the first laser to operate on the principle of converting electrical energy to a laser light output. The first gas laser, the Helium–neon laser (HeNe), was co-invented by Iranian-American engineer and scientist Ali Javan and American physicist William R. Bennett, Jr., in 1960. It produced a coherent light beam in the infrared region of the spectrum at 1.15 micrometres. Types of gas laser Gas lasers using many gases have been built and used for many purposes. Carbon dioxide lasers, or CO2 lasers can emit hundreds of kilowatts at 9.6 µm and 10.6 µm, and are often used in industry for cutting and welding. The efficiency of a CO2 laser is over 10%. Carbon monoxide or "CO" lasers have the potential for very large outputs, but the use of this type of laser is limited by the toxicity of carbon monoxide g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The first laser was built in 1960 by Theodore H. Maiman at Hughes Research Laboratories, based on theoretical work by Charles Hard Townes and Arthur Leonard Schawlow. A laser differs from other sources of light in that it emits light which is ''coherent''. Spatial coherence allows a laser to be focused to a tight spot, enabling applications such as laser cutting and lithography. Spatial coherence also allows a laser beam to stay narrow over great distances (collimation), enabling applications such as laser pointers and lidar (light detection and ranging). Lasers can also have high temporal coherence, which allows them to emit light with a very narrow spectrum. Alternatively, temporal coherence can be used to produce ultrashort pulses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
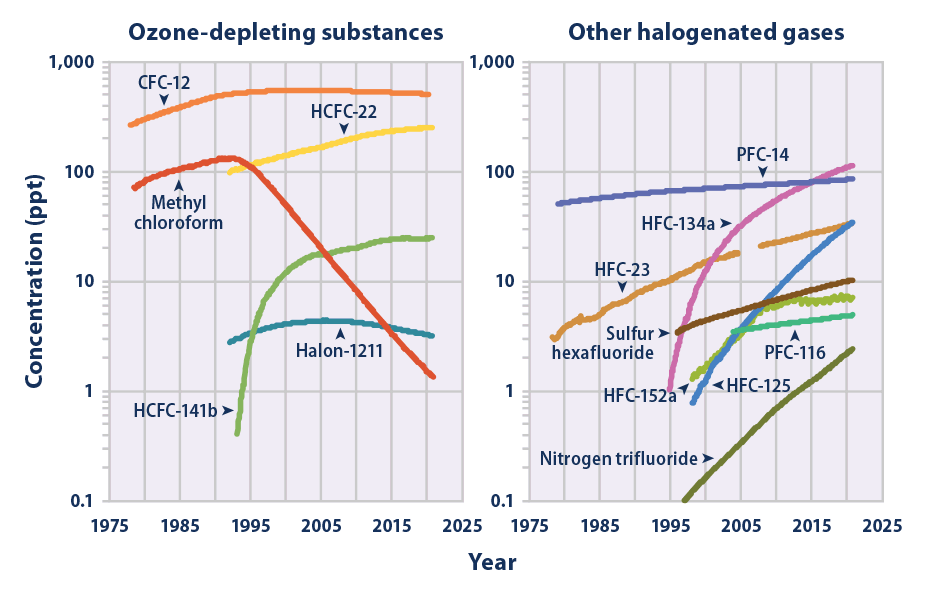
Nitrogen Trifluoride
Nitrogen trifluoride () is an inorganic, colorless, non- flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century. Synthesis and reactivity Nitrogen trifluoride did not exist in significant quantities on Earth prior to its synthesis by humans. It is a rare example of a binary fluoride that can be prepared directly from the elements only at very uncommon conditions, such as an electric discharge. After first attempting the synthesis in 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride. It proved to be far less reactive than the other nitrogen trihalides nitrogen trichloride, ni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. The name neon is derived from the Greek word, , neuter singular form of (), meaning 'new'. Neon is chemically inert, and no uncharged neon compounds are known. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates. During cosmic nucleogenesis of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Although neon is a very ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. The metal is found in the Earth's crust in the pure, free elemental form ("native silver"), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. Most silver is produced as a byproduct of copper, gold, lead, and zinc refining. Silver has long been valued as a precious metal. Silver metal is used in many bullion coins, sometimes alongside gold: while it is more abundant than gold, it is much less abundant as a native metal. Its purity is typically measured on a per-mille basis; a 94%-pure alloy is described as "0.940 fine". As one of the seven metals of antiquity, silver has had an enduring role in most human cultures. Oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling and melting point are the lowest among all the elements. It is the second lightest and second most abundant element in the observable universe (hydrogen is the lightest and most abundant). It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and in Jupiter, due to the very high nuclear binding energy (per nucleon) of helium-4, with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, the vast majority of which was formed during t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Laser
An ion laser is a gas laser that uses an ionized gas as its lasing medium. Like other gas lasers, ion lasers feature a sealed cavity containing the laser medium and mirrors forming a Fabry–Pérot resonator. Unlike helium–neon lasers, the energy level transitions that contribute to laser action come from ions. Because of the large amount of energy required to excite the ionic transitions used in ion lasers, the required current is much greater, and as a result all but the smallest ion lasers are water-cooled. A small air-cooled ion laser might produce, for example, 130 milliwatts of output light with a tube current of about 10 amperes and a voltage of 105 volts. Since one ampere times one volt is one watt, this is an electrical power input of about one kilowatt. Subtracting the (desirable) light output of 130 mW from power input, this leaves the large amount of waste heat of nearly one kW. This has to be dissipated by the cooling system. In other words, the power efficiency ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LASIK
LASIK or Lasik (''laser-assisted in situ keratomileusis''), commonly referred to as laser eye surgery or laser vision correction, is a type of refractive surgery for the correction of myopia, hyperopia, and an actual cure for astigmatism, since it is in the cornea. LASIK surgery is performed by an ophthalmologist who uses a laser or microkeratome to reshape the eye's cornea in order to improve visual acuity. For most people, LASIK provides a long-lasting alternative to eyeglasses or contact lenses. LASIK is very similar to another surgical corrective procedure, photorefractive keratectomy (PRK), and LASEK. All represent advances over radial keratotomy in the surgical treatment of refractive errors of vision. For patients with moderate to high myopia or thin corneas which cannot be treated with LASIK and PRK, the phakic intraocular lens is an alternative. As of 2018, roughly 9.5 million Americans have had LASIK and, globally, between 1991 and 2016, more than 40 million proced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photolithography
In integrated circuit manufacturing, photolithography or optical lithography is a general term used for techniques that use light to produce minutely patterned thin films of suitable materials over a substrate, such as a silicon wafer, to protect selected areas of it during subsequent etching, deposition, or implantation operations. Typically, ultraviolet light is used to transfer a geometric design from an optical mask to a light-sensitive chemical ( photoresist) coated on the substrate. The photoresist either breaks down or hardens where it is exposed to light. The patterned film is then created by removing the softer parts of the coating with appropriate solvents. Conventional photoresists typically consists of three components: resin, sensitizer, and solvent. Photolithography processes can be classified according to the type of light used, such as ultraviolet, deep ultraviolet, extreme ultraviolet, or X-ray. The wavelength of light used determines the minimum feature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionize atoms, it can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surfaces with which it comes into contact. F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excited State
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: spontaneous or induced emission of a quantum of energy (such as a photon or a phonon) usually occurs shortly after the system is promoted to the excited state, returning the system to a state with lower energy (a less excited state or the ground state). This return to a lower energy level is often loosely described as decay and is the inverse of excitation. Long-lived excited states are often calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |