|
Growth Cone
A growth cone is a large actin-supported extension of a developing or regenerating neurite seeking its synaptic target. It is the growth cone that drives axon growth. Their existence was originally proposed by Spanish histologist Santiago Ramón y Cajal based upon stationary images he observed under the microscope. He first described the growth cone based on fixed cells as "a concentration of protoplasm of conical form, endowed with amoeboid movements" (Cajal, 1890). Growth cones are situated on the tips of neurites, either dendrites or axons, of the nerve cell. The sensory, motor, integrative, and adaptive functions of growing axons and dendrites are all contained within this specialized structure. Structure The morphology of the growth cone can be easily described by using the hand as an analogy. The fine extensions of the growth cone are pointed filopodia known as microspikes. The filopodia are like the "fingers" of the growth cone; they contain bundles of actin filame ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrin
Integrins are transmembrane receptors that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface (''e.g''. signal platelets to initiate an interaction with coagulation factors). Several types of integrins exist, and one cell generally has multiple different types on its surface. Integrins are found in all animals while integrin-like receptors are found in plant cells. Integrins work alongside other proteins such as cadherins, the immunoglobulin superfamily cell adhesion molecules, selectins and syndecans, to mediate cell–cell and cell–matrix interaction. Ligands for integrins include fibronectin, vitronectin, collagen and laminin. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basal Lamina
The basal lamina is a layer of extracellular matrix secreted by the epithelial cells, on which the epithelium sits. It is often incorrectly referred to as the basement membrane, though it does constitute a portion of the basement membrane. The basal lamina is visible only with the electron microscope, where it appears as an electron-dense layer that is 20–100 nm thick (with some exceptions that are thicker, such as basal lamina in lung alveoli and renal glomeruli). Structure The layers of the basal lamina ("BL") and those of the basement membrane ("BM") are described below: Anchoring fibrils composed of type VII collagen extend from the basal lamina into the underlying reticular lamina and loop around collagen bundles. Although found beneath all basal laminae, they are especially numerous in stratified squamous cells of the skin. These layers should not be confused with the lamina propria, which is found outside the basal lamina. Basement membrane The basement memb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
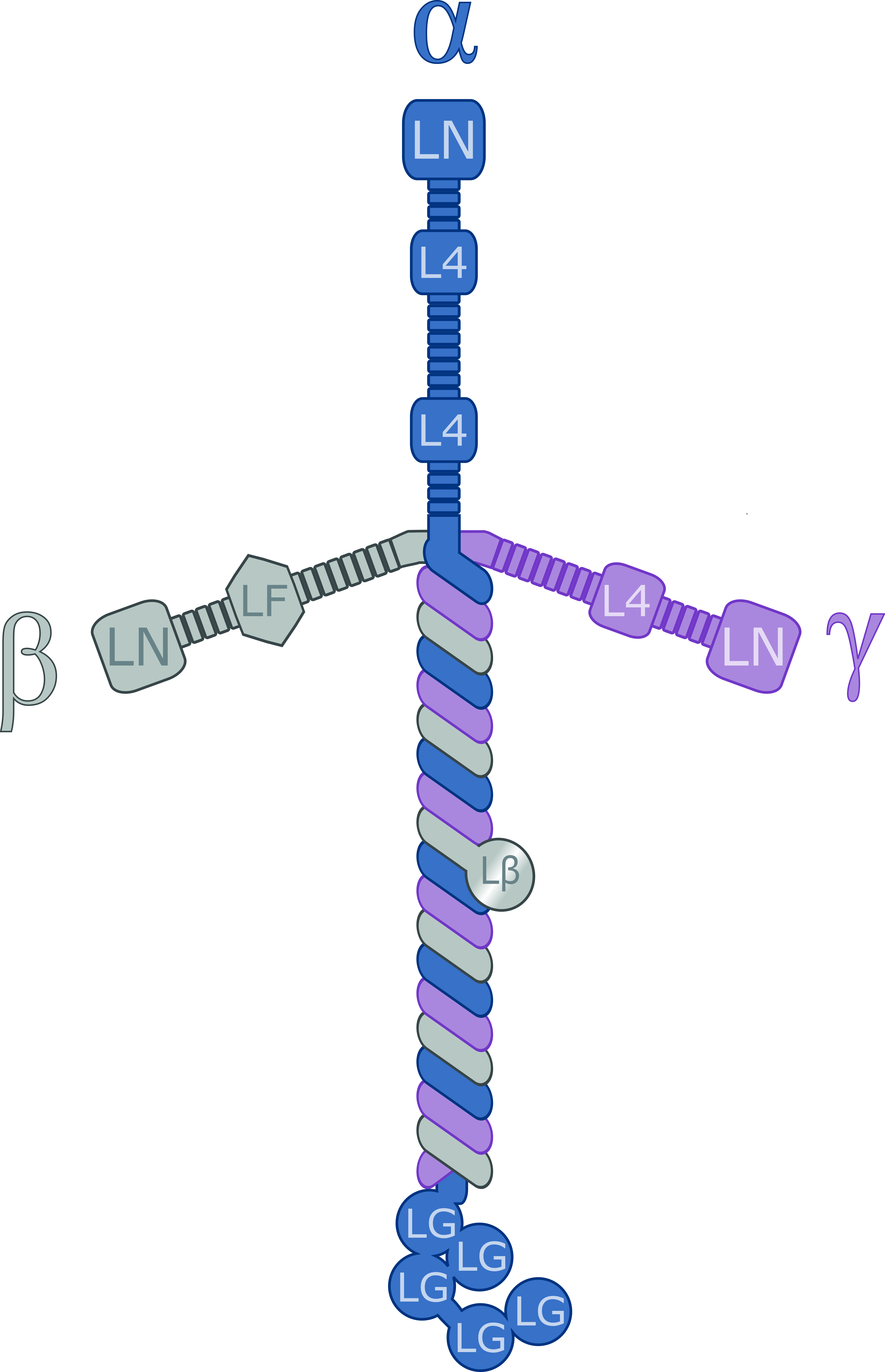
Laminin
Laminins are a family of glycoproteins of the extracellular matrix of all animals. They are major components of the basal lamina (one of the layers of the basement membrane), the protein network foundation for most cells and organs. The laminins are an important and biologically active part of the basal lamina, influencing cell differentiation, migration, and adhesion. Laminins are heterotrimeric proteins with a high molecular mass (~400 to ~900 kDa). They contain three different chains (α, β and γ) encoded by five, four, and three paralogous genes in humans, respectively. The laminin molecules are named according to their chain composition. Thus, laminin-511 contains α5, β1, and γ1 chains. Fourteen other chain combinations have been identified ''in vivo''. The trimeric proteins intersect to form a cross-like structure that can bind to other cell membrane and extracellular matrix molecules. The three shorter arms are particularly good at binding to other laminin molecule ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MDia1
mDia1 (also known as Dia1, Drf1 for Diaphanous-related formin-1, Diaph1, KIAA4062, p140mDia, mKIAA4062, or D18Wsu154e) is a member of the protein family called the formins and is a Rho effector. It is the mouse version of the diaphanous homolog 1 of Drosophila. mDia1 localizes to cells' mitotic spindle and midbody, plays a role in stress fiber and filopodia formation, phagocytosis, activation of serum response factor, formation of adherens junctions, and it can act as a transcription factor. mDia1 accelerates actin nucleation and elongation by interacting with barbed ends (fast-growing ends) of actin filaments. The gene encoding mDia1 is located on Chromosome 18 of Mus musculus and named ''Diap1''. mDia1 is highly homologous to Drosophila diaphanous, regulating the cytokinetic ring during cytokinesis. Homologues in other species are known as well, like the human DIAP1, budding yeast Bni1 or fission yeast Cdc12p. The gene has been knocked-out in mice. Structure The product o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in ''Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Talin Protein
Talin is a high-molecular-weight cytoskeletal protein concentrated at regions of cell–substratum contact and, in lymphocytes, at cell–cell contacts. Discovered in 1983 by Keith Burridge and colleagues, talin is a ubiquitous cytosolic protein that is found in high concentrations in focal adhesions. It is capable of linking integrins to the actin cytoskeleton either directly or indirectly by interacting with vinculin and α-actinin. Also, talin-1 drives extravasation mechanism through engineered human microvasculature in microfluidic systems. Talin-1 is involved in each part of extravasation affecting adhesion, trans-endothelial migration and the invasion stages. Integrin receptors are involved in the attachment of adherent cells to the extracellular matrix and of lymphocytes to other cells. In these situations, talin codistributes with concentrations of integrins in the plasma membrane. Furthermore, in vitro binding studies suggest that integrins bind to talin, althoug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filamin
Filamins are a class of proteins that hold two actin filaments at large angles. Filamin protein in mammals is made up of an actin-binding domain at its N-terminus that is followed by 24 immunoglobulin-like repeat modules of roughly 95 amino acids. There are two hinge regions; between repeats 15-16 and 23-24. Filamin gets cleaved at these hinge regions to generate smaller fragments of the protein. Filamin has two actin-binding sites with a V-linkage between them, so that it cross-links actin filaments into a network with the filaments orientated almost at right angles to one another. Filamin proteins include: * FLNA * FLNB * FLNC Over-expression of FLNA stops the regeneration of bladder carcinoma (BC) cells, by inhibiting the cell cycle The cell cycle, or cell-division cycle, is the series of events that take place in a cell that cause it to divide into two daughter cells. These events include the duplication of its DNA ( DNA replication) and some of its organelles, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fascin
Fascin is an actin bundling protein. Species and tissue distribution It is a 54-58 kilodalton monomeric actin filament bundling protein originally isolated from sea urchin egg but also found in ''Drosophila'' and vertebrates, including humans. Fascin (from the Latin for ''bundle'') is spaced at 11 nanometre intervals along the filament. The bundles in cross section are seen to be hexagonally packed, and the longitudinal spacing is compatible with a model where fascin cross-links at alternating 4 and 5 actins. It is calcium insensitive and monomeric. Three forms of fascin are found in vertebrates: Fascin1, widely found in the nervous system and elsewhere; fascin2 found in the retinal photoreceptor cells; fascin3, which is only found in the testes. Function Fascin binds beta-catenin, and colocalizes with it at the leading edges and borders of epithelial and endothelial cells. The role of Fascin in regulating cytoskeletal structures for the maintenance of cell ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteomes
The proteome is the entire set of proteins that is, or can be, expressed by a genome, cell, tissue, or organism at a certain time. It is the set of expressed proteins in a given type of cell or organism, at a given time, under defined conditions. Proteomics is the study of the proteome. Types of proteomes While proteome generally refers to the proteome of an organism, multicellular organisms may have very different proteomes in different cells, hence it is important to distinguish proteomes in cells and organisms. A cellular proteome is the collection of proteins found in a particular cell type under a particular set of environmental conditions such as exposure to hormone stimulation. It can also be useful to consider an organism's complete proteome, which can be conceptualized as the complete set of proteins from all of the various cellular proteomes. This is very roughly the protein equivalent of the genome. The term ''proteome'' has also been used to refer to the collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |