|
Grieco Elimination
The Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene. It is named for Paul Grieco. The alcohol first reacts with ''o''-nitrophenylselenocyanate and tributylphosphine to form a selenide via a nucleophilic substitution on the electron-deficient selenium. In the second step, the selenide is oxidized with hydrogen peroxide to give a selenoxide. This structure decomposes to form an alkene by an Ei elimination mechanism with expulsion of a selenol in a fashion similar to that of the Cope elimination The Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of the N-oxide to form an alkene and a hydroxylamine. Mechanism and applications The reaction mechanism involves an intramolecular 5-membered cyclic .... This reaction takes part in the synthesis of ring C of the Danishefsky Taxol synthesis. References * ''Organoselenium chemistry. A facil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elimination Reactions
Elimination may refer to: Science and medicine *Elimination reaction, an organic reaction in which two functional groups split to form an organic product *Bodily waste elimination, discharging feces, urine, or foreign substances from the body via defecation, urination, and emesis *Drug elimination, clearance of a drug or other foreign agent from the body *Elimination, the destruction of an infectious disease in one region of the world as opposed to its eradication from the entire world *Hazard elimination, the most effective type of hazard control * Elimination (pharmacology), processes by which a drug is eliminated from an organism Logic and mathematics * Elimination theory, the theory of the methods to eliminate variables between polynomial equations. * Disjunctive syllogism, a rule of inference * Gaussian elimination, a method of solving systems of linear equations * Fourier–Motzkin elimination, an algorithm for reducing systems of linear inequalities * Process of elim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Danishefsky Taxol Total Synthesis
The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis. Danishefsky's route to Taxol has many similarities with that of Nicolaou. Both are examples of convergent synthesis with a coupling of the A and the C ring from two precursors. The main characteristic of the Danishefsky variant is the completion of the oxetane D ring onto the cyclohexanol C ring prior to the construction of the 8-membered B ring. The most prominent starting material is the (+) enantiomer of the Wieland-Miescher ketone. This compound is commercially available as a single enantiomer and the single chiral group present in this molecule is able to drive the entire sequence of organic reactions to a sin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cope Elimination
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of the N-oxide to form an alkene and a hydroxylamine. Mechanism and applications The reaction mechanism involves an intramolecular 5-membered cyclic transition state, leading to a ''syn'' elimination product, an Ei pathway. This organic reaction is closely related to the Hofmann elimination, but the base is a part of the leaving group. The amine oxide is prepared by oxidation of the corresponding amine with an oxidant such as meta-chloroperoxybenzoic acid (''m''CPBA). The actual elimination just requires heat. : Illustrative of the Cope reaction is a synthesis of methylenecyclohexane: : Piperidines are resistant to an intramolecular Cope reaction but with pyrrolidine and with rings of size 7 and larger, the reaction product is an unsaturated hydroxyl amine. This result is consistent with the 5-membered cyclic transition state. : Reverse reaction The reverse or retro-Cope elimi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
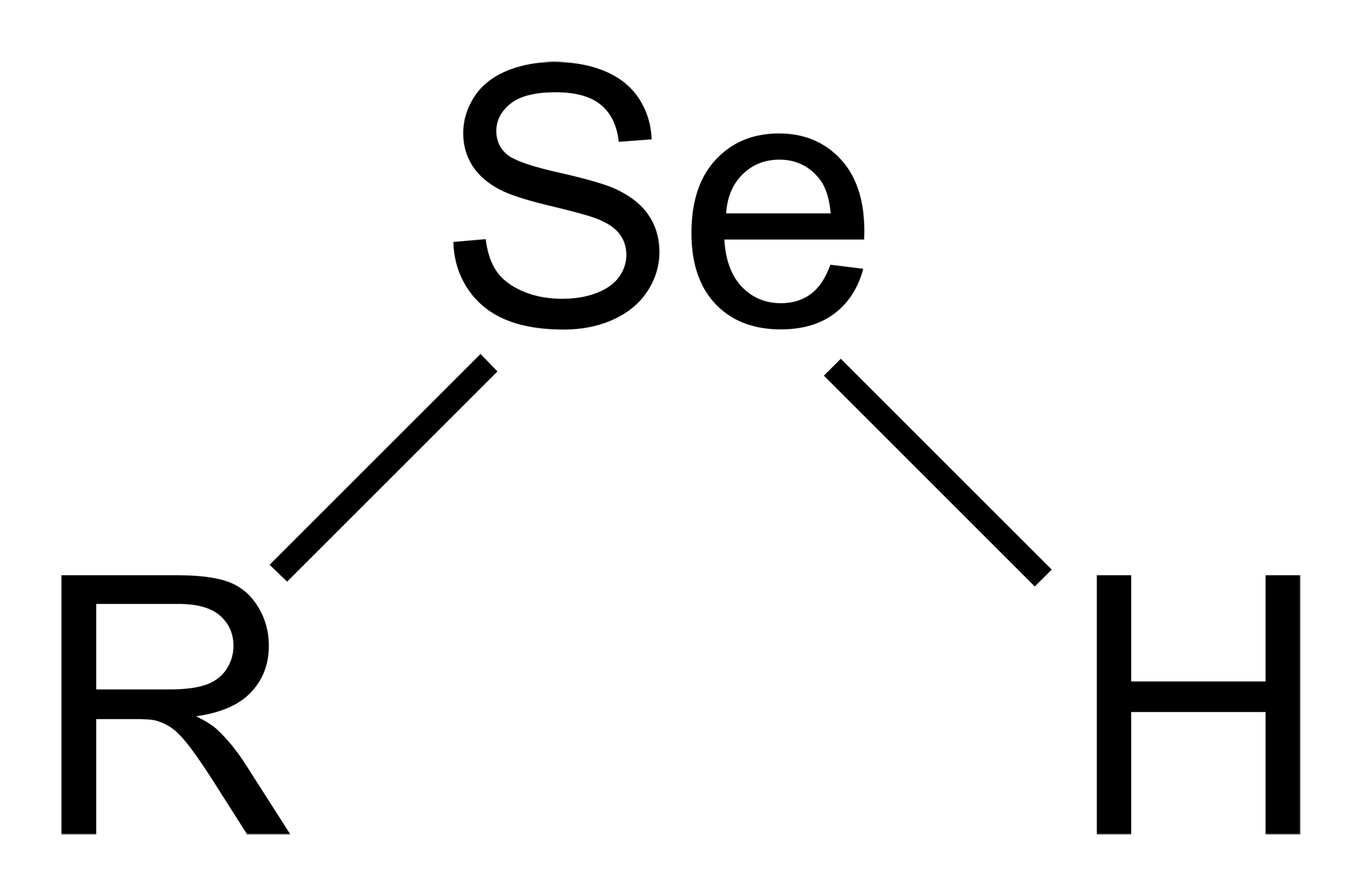
Selenol
Selenols are organic compounds that contain the functional group with the connectivity C–Selenium, Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. The best known member of the group is the amino acid selenocysteine. Structure, bonding, properties Selenols are structurally similar to thiols, but the C-Se bond is about 8% longer at 196 pm. The C–Se–H angle approaches 90°. The bonding involves almost pure p-orbitals on Se, hence the near 90 angles. The Se–H bond energy is weaker than the S–H bond, consequently selenols are easily oxidized and serve as H-atom donors. The Se-H bond is much weaker than the S-H bond as reflected in their respective bond dissociation energy (BDE). For C6H5Se-H, the BDE is 326 kJ/mol, while for C6H5S-H, the BDE is 368 kJ/mol. Selenol acids are about 1000 times stronger than thiols: the p''K''a of CH3SeH is 5.2 vs 8.3 for CH3SH. Deprotonation affo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ei Mechanism
In organic chemistry, the Ei mechanism (Elimination Internal/Intramolecular), also known as a thermal syn elimination or a pericyclic syn elimination, is a special type of elimination reaction in which two Vicinal (chemistry), vicinal (adjacent) substituents on an alkane framework leave simultaneously via a cyclic transition state to form an alkene in a syn elimination, ''syn'' elimination. This type of elimination is unique because it is thermally activated and does not require additional reagents, unlike regular eliminations, which require an acid or Base (chemistry), base, or would in many cases involve charged Reaction intermediate, intermediates. This reaction mechanism is often found in pyrolysis. General features Compounds that undergo elimination through cyclic transition states upon heating, with no other reagents present, are given the designation as Ei reactions. Depending on the compound, elimination takes place through a four, five, or six-membered transition state.Ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenoxide
Organoselenium compounds (or seleno-organic) are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments. Selenium can exist with oxidation state −2, +2, +4, +6. Se(II) is the dominant form in organoselenium chemistry. Down the group 16 column, the bond strength becomes increasingly weaker (234 kJ/ mol for the C−Se bond and 272 kJ/mol for the C−S bond) and the bond lengths longer (C−Se 198 pm, C−S 181 pm and C−O 141 pm). Selenium compounds are more nucleophilic than the corresponding sulfur compounds and also more acidic. The p''K''a values of XH2 are 16 for oxygen, 7 for sulfur and 3.8 for selenium. In contrast to sulfoxides, the corresponding se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium – from Greek ( 'Moon') – was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in metal sulfide ores, where it partially replaces the sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores, most often during production. Minerals that are pure selenide or selenate compounds are known but rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, have been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organic Photochemistry, photochemical reactions and organic redox reaction, redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :R-Br + OH- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tributylphosphine
Tributylphosphine is the organophosphorus compound with the formula P(CH). Abbreviated or PBu, it is a tertiary phosphine. It is an oily liquid at room temperature, with a nauseating odor. It reacts slowly with atmospheric oxygen, and rapidly with other oxidizing agents, to give the corresponding phosphine oxide. It is usually handled using air-free techniques. Preparation Tributylphosphine is prepared industrially by the hydrophosphination of phosphine with butene: the addition proceeds by a free radical mechanism, and so the Markovnikov rule is not followed. :PH + 3CH=CHCHCH → P(CHCHCHCH) Tributylphosphine can be prepared in the laboratory by reaction of the appropriate Grignard reagent with phosphorus trichloride although, as it is commercially available at reasonable prices, it is rare to have to perform the small-scale preparation. :3 BuMgCl + PCl → PBu + 3 MgCl Reactions Tributylphosphine reacts with oxygen to give the phosphine oxide: :2 PBu3 + O2 → 2 OPBu3 Because ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |