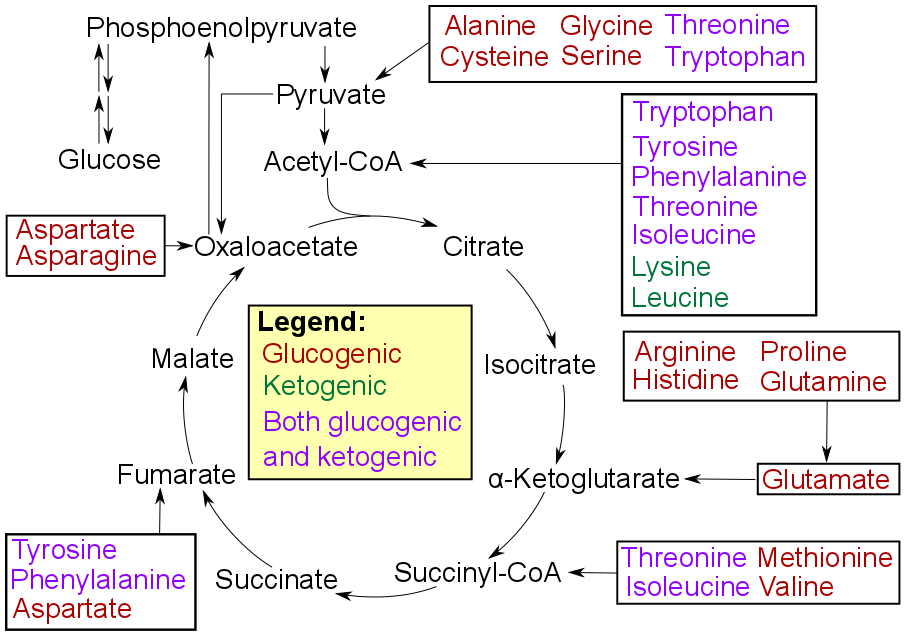
|
Glycolysis Reaction
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. Glycolysis is a metabolic pathway that does not require oxygen (In anaerobic conditions pyruvate is converted to lactic acid). The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, occur in the oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal. In most organisms, glycolysis occurs in the liquid part of cells, the cytosol. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerobic Respiration Summary
Aerobic means "requiring air," in which "air" usually means oxygen. Aerobic may also refer to * Aerobic exercise, prolonged exercise of moderate intensity * Aerobics, a form of aerobic exercise * Aerobic respiration, the aerobic process of cellular respiration * Aerobic organism Aerobic means "requiring air," in which "air" usually means oxygen. Aerobic may also refer to * Aerobic exercise, prolonged exercise of moderate intensity * Aerobics, a form of aerobic exercise * Aerobic respiration, the aerobic process of cell ..., a living thing with an oxygen-based metabolism See also * Anaerobic (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geoffrey Michael Gadd
Geoffrey Michael Gadd (born 15 July 1954) is a British-Irish microbiologist and mycologist specializing in geomicrobiology, geomycology, and bioremediation. He is currently a professor at the University of Dundee, holding the Boyd Baxter Chair of Biology, and is head of the Geomicrobiology Group. Gadd contributes to his field's vitality via contributions to many professional societies and national and international editorial, advisory groups, and committees. He was President of the British Mycological Society (2004-2007), is currently Treasurer (2008-) and a member of several BMS committees. He served the former Society for General Microbiology (now Microbiology Society) as Convenor of the Environmental Microbiology group (2005-2008) and was the first Chair of the Eukaryotic Division (2008-2010). He also served on the Cell Biology; Physiology and Biochemistry; and Environmental Microbiology Groups and organized several SGM meetings and Main symposia. He has given invited lecture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogenolysis
Glycogenolysis is the breakdown of glycogen (n) to glucose-1-phosphate and glycogen (n-1). Glycogen branches are catabolized by the sequential removal of glucose monomers via phosphorolysis, by the enzyme glycogen phosphorylase. Mechanism The overall reaction for the breakdown of glycogen to glucose-1-phosphate is: : glycogen(n residues) + Pi glycogen(n-1 residues) + glucose-1-phosphate Here, glycogen phosphorylase cleaves the bond linking a terminal glucose residue to a glycogen branch by substitution of a phosphoryl group for the α →4linkage. Glucose-1-phosphate is converted to glucose-6-phosphate (which often ends up in glycolysis) by the enzyme phosphoglucomutase. Glucose residues are phosphorolysed from branches of glycogen until four residues before a glucose that is branched with a α →6linkage. Glycogen debranching enzyme then transfers three of the remaining four glucose units to the end of another glycogen branch. This exposes the α →6branching point, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogenesis
Glycogenesis is the process of glycogen synthesis, in which glucose molecules are added to chains of glycogen for storage. This process is activated during rest periods following the Cori cycle, in the liver, and also activated by insulin in response to high glucose levels. Steps *Glucose is converted into glucose 6-phosphate by the action of glucokinase or hexokinase with conversion of ATP to ADP. * Glucose-6-phosphate is converted into glucose-1-phosphate by the action of phosphoglucomutase, passing through the obligatory intermediate glucose-1,6-bisphosphate. * Glucose-1-phosphate is converted into UDP-glucose by the action of the enzyme UDP-glucose pyrophosphorylase. Pyrophosphate is formed, which is later hydrolysed by pyrophosphatase into two phosphate molecules. * The enzyme glycogenin is needed to create initial short glycogen chains, which are then lengthened and branched by the other enzymes of glycogenesis. Glycogenin, a homodimer, has a tyrosine residue on each ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen ( glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise. In humans, substrates for gluconeogenesis may come from any non-carbohydrate sources that can be converted to pyruvate or intermediates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monosaccharide
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. They are usually colorless, water-soluble, and crystalline solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides). Examples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose. Each carbon atom that supports a hydroxyl group is chiral, except those at the end of the chain. This gives rise to a number of isomeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substrate-level Phosphorylation
Substrate-level phosphorylation is a metabolism reaction that results in the production of ATP or GTP by the transfer of a phosphate group from a substrate directly to ADP or GDP. Transferring from a higher energy (whether phosphate group attached or not) into a lower energy product. This process uses some of the released chemical energy, the Gibbs free energy, to transfer a phosphoryl (PO3) group to ADP or GDP from another phosphorylated compound. Occurs in glycolysis and in the citric acid cycle. Unlike oxidative phosphorylation, oxidation and phosphorylation are not coupled in the process of substrate-level phosphorylation, and reactive intermediates are most often gained in the course of oxidation processes in catabolism. Most ATP is generated by oxidative phosphorylation in aerobic or anaerobic respiration while substrate-level phosphorylation provides a quicker, less efficient source of ATP, independent of external electron acceptors. This is the case in human erythro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell. Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or to fatty acids through a reaction with acetyl-CoA. It can also be used to construct the amino acid alanine and can be converted into ethanol or lactic acid via fermentation. Pyruvic acid supplies energy to cells through the citric acid cycle (also known as the Krebs cycle) when oxygen is present (aerobic respiration), and alternatively ferments to produce lactate when oxygen is lacking. Chemistry In 1834, Théophile-Jules Pelouze distilled tartaric acid and isolated glutaric acid and another unknown organic acid. Jöns Jacob Berzelius characterized this other acid the following year and named pyruvic acid because it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerobic Respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, releasing energy. Respiration is one of the key ways a cell releases chemical energy to fuel cellular activity. The overall reaction occurs in a series of biochemical steps, some of which are redox reactions. Although cellular respiration is technically a combustion reaction, it is an unusual one because of the slow, controlled release of energy from the series of reactions. Nutrients that are commonly used by animal and pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactic Acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate (or the lactate anion). The name of the derived acyl group is lactoyl. In solution, it can ionize by loss of a proton to produce the lactate ion . Compared to acetic acid, its p''K'' is 1 unit less, meaning lactic acid is ten times more acidic than acetic acid. This higher acidity is the consequence of the intramolecular hydrogen bonding between the α-hydroxyl and the carboxylate group. Lactic acid is chiral, consisting of two enantiomers. One ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermentation (biochemistry)
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage. The science of fermentation is known as zymology. In microorganisms, fermentation is the primary means of producing adenosine triphosphate (ATP) by the degradation of organic nutrients anaerobically. Humans have used fermentation to produce foodstuffs and beverages since the Neolithic age. For example, fermentation is used for preservation in a process that produces lactic acid found in such sour foods as pickled cucumbers, kombucha, kimchi, and yogurt, as well as for producing alcoholic beverages such as wine and beer. Fermentation also occurs within the gastrointestinal tracts of all an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |