|
Glycerol Kinase
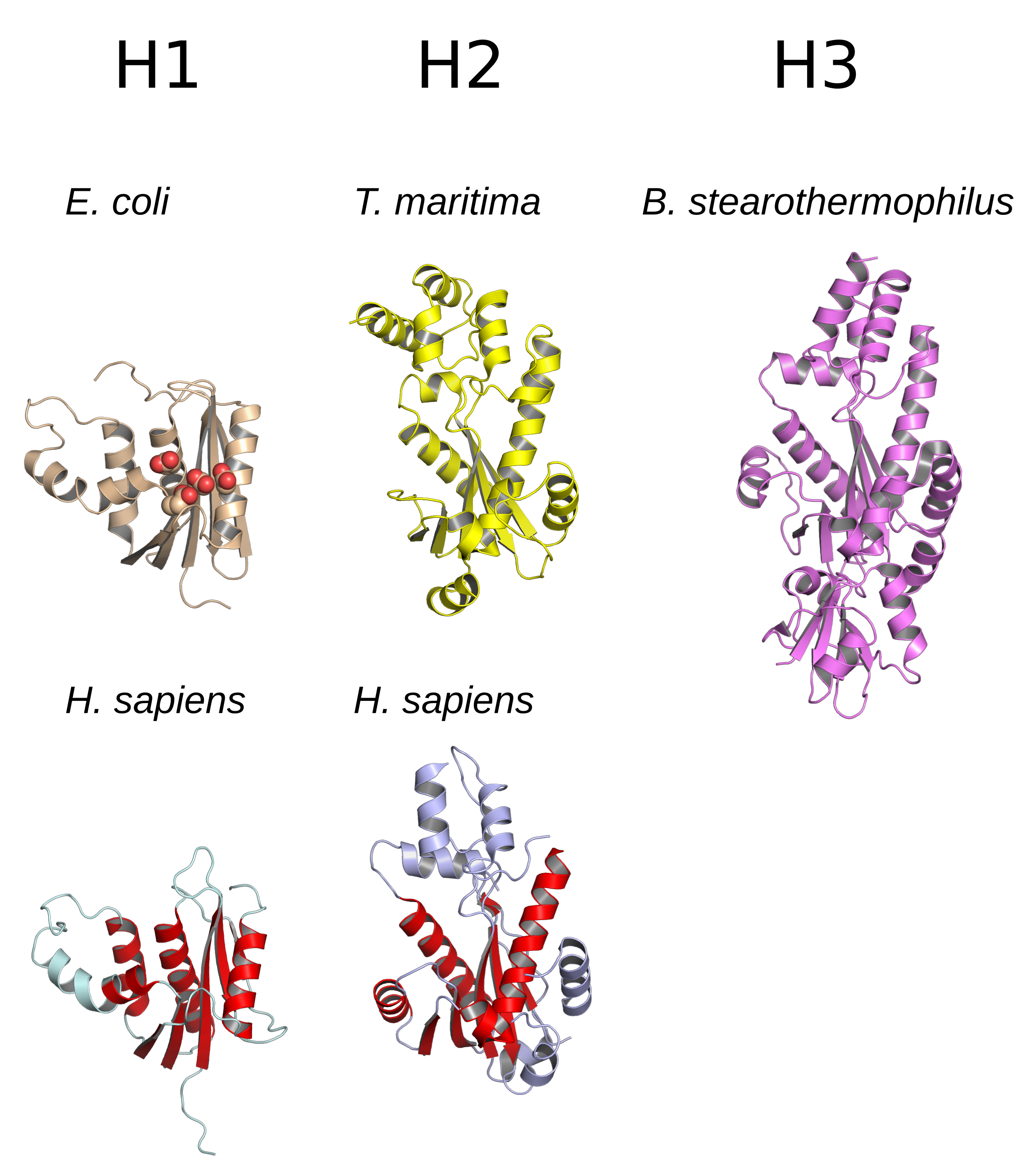
Glycerol kinase, encoded by the gene ''GK'', is a phosphotransferase enzyme involved in triglycerides and glycerophospholipids synthesis. Glycerol kinase catalyzes the transfer of a phosphate from ATP to glycerol thus forming glycerol 3-phosphate: :ATP + glycerol ADP + ''sn''-glycerol 3-phosphate Adipocytes lack glycerol kinase so they cannot metabolize the glycerol produced during triacyl glycerol degradation. This glycerol is instead shuttled to the liver via the blood where it is: * Phosphorylated by glycerol kinase to glycerol 3-phosphate. * Converted from glycerol 3-phosphate to dihydroxyacetone phosphate (DHAP) via glycerol 3-phosphate dehydrogenase. DHAP can participate in glycolysis or gluconeogenesis. Enzyme regulation This protein may use the morpheein model of allosteric regulation. Structure Glycerol Kinase (alternative name, ATP:glycerol 3-phosphotransferase or Glycerokinase) adopts a ribonuclease H-like fold consisting of an alpha-beta 2-layer sandwich of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphotransferase
Phosphotransferases are a category of enzymes ( EC number 2.7) that catalyze phosphorylation reactions. The general form of the reactions they catalyze is: :A-P + B \rightleftharpoons B-P + A Where ''P'' is a phosphate group and A and B are the donating and accepting molecules, respectively. Classification Phosphotransferases are generally classified according to the acceptor molecule. , Classification in this article follows the rules of Enzyme Nomenclature of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). *EC 2.7.1 Phosphotransferases with an as acceptor *EC 2.7.2 Phosphotransferases with a carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol-3-phosphate Dehydrogenase
''sn''-Glycerol 3-phosphate is the organic ion with the formula HOCH2CH(OH)CH2OPO32-. It is one of three stereoisomers of the ester of dibasic phosphoric acid (HOPO32-) and glycerol. It is a component of glycerophospholipids. Equally appropriate names in biochemical context include glycerol-3-phosphate, 3-''O''-phosphonoglycerol, 3-phosphoglycerol; and Gro3P. From a historical reason, it is also known as -glycerol 3-phosphate, -glycerol 1-phosphate, -α-glycerophosphoric acid. Biosynthesis Glycerol 3-phosphate is synthesized by reducing dihydroxyacetone phosphate (DHAP), an intermediate in glycolysis. The reduction is catalyzed by glycerol-3-phosphate dehydrogenase. DHAP and thus glycerol 3-phosphate can also be synthesized from amino acids and citric acid cycle intermediates via the glyceroneogenesis pathway. : + NAD(P)H + H+ → + NAD(P)+ It is also synthesized by the phosphorylation of glycerol, which is generated by hydrolysis of fats. This esterification is catal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CATH
The CATH Protein Structure Classification database is a free, publicly available online resource that provides information on the evolutionary relationships of protein domains. It was created in the mid-1990s by Professor Christine Orengo and colleagues including Janet Thornton and David Jones, and continues to be developed by the Orengo group at University College London. CATH shares many broad features with the SCOP resource, however there are also many areas in which the detailed classification differs greatly. Hierarchical organization Experimentally-determined protein three-dimensional structures are obtained from the Protein Data Bank and split into their consecutive polypeptide chains, where applicable. Protein domains are identified within these chains using a mixture of automatic methods and manual curation. The domains are then classified within the CATH structural hierarchy: at the Class (C) level, domains are assigned according to their secondary structure content, i. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rnase H
Ribonuclease H (abbreviated RNase H or RNH) is a family of non-sequence-specific endonuclease enzymes that catalyze the cleavage of RNA in an RNA/ DNA substrate via a hydrolytic mechanism. Members of the RNase H family can be found in nearly all organisms, from bacteria to archaea to eukaryotes. The family is divided into evolutionarily related groups with slightly different substrate preferences, broadly designated ribonuclease H1 and H2. The human genome encodes both H1 and H2. Human ribonuclease H2 is a heterotrimeric complex composed of three subunits, mutations in any of which are among the genetic causes of a rare disease known as Aicardi–Goutières syndrome. A third type, closely related to H2, is found only in a few prokaryotes, whereas H1 and H2 occur in all domains of life. Additionally, RNase H1-like retroviral ribonuclease H domains occur in multidomain reverse transcriptase proteins, which are encoded by retroviruses such as HIV and are required for viral r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric Regulation
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site. The site to which the effector binds is termed the ''allosteric site'' or ''regulatory site''. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change and/or a change in protein dynamics. Effectors that enhance the protein's activity are referred to as ''allosteric activators'', whereas those that decrease the protein's activity are called ''allosteric inhibitors''. Allosteric regulations are a natural example of control loops, such as feedback from downstream products or feedforward from upstream substrates. Long-range allostery is especially important in cell signaling. Allosteric regulation is also particularly important in the cell's ability to adjust enzyme activity. The term ''allostery'' comes from the Ancient Greek ''allos'' (), "other", and ''stereos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morpheein
Morpheeins are proteins that can form two or more different homo-oligomers (morpheein forms), but must come apart and change shape to convert between forms. The alternate shape may reassemble to a different oligomer. The shape of the subunit dictates which oligomer is formed. Each oligomer has a finite number of subunits (stoichiometry). Morpheeins can interconvert between forms under physiological conditions and can exist as an equilibrium of different oligomers. These oligomers are physiologically relevant and are not misfolded protein; this distinguishes morpheeins from prions and amyloid. The different oligomers have distinct functionality. Interconversion of morpheein forms can be a structural basis for allosteric regulation, an idea noted many years ago, and later revived. A mutation that shifts the normal equilibrium of morpheein forms can serve as the basis for a conformational disease. Features of morpheeins can be exploited for drug discovery. The dice image (F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
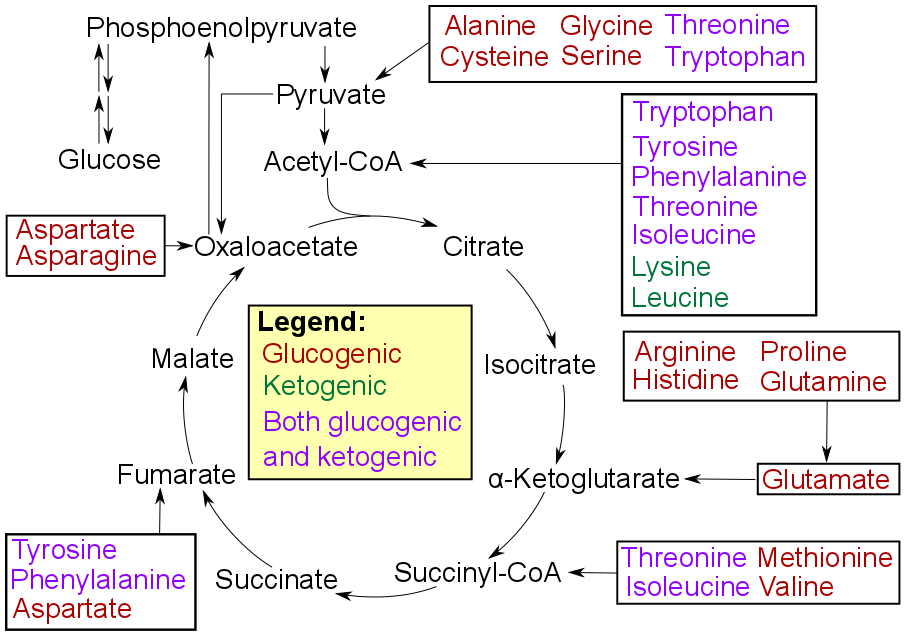
Gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non- carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen ( glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels ( hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise. In humans, substrates for gluconeogenesis may come from any non-carbohydrate sources that can be converted to pyruvate or in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. Glycolysis is a metabolic pathway that does not require oxygen (In anaerobic conditions pyruvate is converted to lactic acid). The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, occur in the oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal. In most organisms, glycolysis occurs in the liquid part of cells, the cytosol. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Ka ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydroxyacetone Phosphate
Dihydroxyacetone phosphate (DHAP, also glycerone phosphate in older texts) is the anion with the formula HOCH2C(O)CH2OPO32-. This anion is involved in many metabolic pathways, including the Calvin cycle in plants and glycolysis.Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. . It is the phosphate ester of dihydroxyacetone. Role in glycolysis Dihydroxyacetone phosphate lies in the glycolysis metabolic pathway, and is one of the two products of breakdown of fructose 1,6-bisphosphate, along with glyceraldehyde 3-phosphate. It is rapidly and reversibly isomerised to glyceraldehyde 3-phosphate. ''The numbering of the carbon atoms indicates the fate of the carbons according to their position in fructose 6-phosphate.'' Role in other pathways In the Calvin cycle, DHAP is one of the products of the sixfold reduction of 1,3-bisphosphoglycerate by NADPH. It is also used in the synthesis of sedoheptulose 1,7-bisphosph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the circulatory system is also known as ''peripheral blood'', and the blood cells it carries, ''peripheral blood cells''. Blood is composed of blood cells suspended in blood plasma. Plasma, which constitutes 55% of blood fluid, is mostly water (92% by volume), and contains proteins, glucose, mineral ions, hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and blood cells themselves. Albumin is the main protein in plasma, and it functions to regulate the colloidal osmotic pressure of blood. The blood cells are mainly red blood cells (also called RBCs or erythrocytes), white blood cells (also called WBCs or leukocytes) and platelets (also called thrombocytes). The most abundant cells in vertebrate blood a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |