|
Gas Thermometer
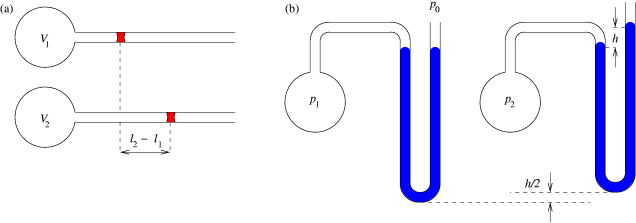
A gas thermometer is a thermometer that measures temperature by the variation in volume or pressure of a gas. Volume Thermometer This thermometer functions by Charles's Law. Charles's Law states that when the temperature of a gas increases, so does the volume. Using Charles's Law, the temperature can be measured by knowing the volume of gas at a certain temperature by using the formula, written below. Translating it to the correct levels of the device that is holding the gas. This works on the same principle as mercury thermometers. :V \propto T\, or :\frac=k V is the volume, T is the thermodynamic temperature, k is the constant for the system. k is not a fixed constant across all systems and therefore needs to be found experimentally for a given system through testing with known temperature values. Pressure Thermometer and Absolute Zero The constant volume gas thermometer plays a crucial role in understanding how absolute zero could be discovered long before the adve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermometer
A thermometer is a device that temperature measurement, measures temperature or a temperature gradient (the degree of hotness or coldness of an object). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb of a mercury-in-glass thermometer or the pyrometric sensor in an infrared thermometer) in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value (e.g. the visible scale that is marked on a mercury-in-glass thermometer or the digital readout on an infrared model). Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research. History While an individual thermometer is able to measure degrees of hotness, the readings on two thermometers cannot be compared unless they conform to an agreed scale. Today there is an absolute thermodynamic temperature scale. Internationally agreed temperature scales are designed to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), the latter being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible to extract energy as heat from a body at that temperature. Temperature is important in all fields of natur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles's Law
Charles's law (also known as the law of volumes) is an experimental gas law that describes how gases tend to expand when heated. A modern statement of Charles's law is: When the pressure on a sample of a dry gas is held constant, the Kelvin temperature and the volume will be in direct proportion. This relationship of direct proportion can be written as: :V \propto T So this means: :\frac = k, \quad \text \quad V=k T where: * is the volume of the gas, * is the temperature of the gas (measured in kelvins), and * is a non-zero constant. This law describes how a gas expands as the temperature increases; conversely, a decrease in temperature will lead to a decrease in volume. For comparing the same substance under two different sets of conditions, the law can be written as: \frac=\frac The equation shows that, as absolute temperature increases, the volume of the gas also increases in proportion. History The law was named after scientist Jacques Charles, who formulated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics. Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic work and heat transfer as defined in thermodynamics, but the kelvin was redefined by international agreement in 2019 in terms of phenomena that are now understood as manifestations of the kinetic energy of free motion of microscopic particles such as atoms, molecules, and electrons. From the thermodynamic viewpoint, for historical reasons, because of how it is defined and measured, this microscopic kinetic definition is regarded as an "empirical" temperature. It was adopted because in practice it can generally be measured more precisely than can Kelvin's thermodynamic temperature. A thermodynamic temperature reading of zero is of particular importance for the third law of thermodynamics. By convention, it is reported on the ''Kelvin scale'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Thermometer And Absolute Zero
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture, such as air, contains a variety of pure gases. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colourless gas invisible to the human observer. The gaseous state of matter occurs between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention. High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gases or Fermi gases. For a comprehensive listi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absolute Zero
Absolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvin. The fundamental particles of nature have minimum vibrational motion, retaining only quantum mechanical, zero-point energy-induced particle motion. The theoretical temperature is determined by extrapolating the ideal gas law; by international agreement, absolute zero is taken as −273.15 degrees on the Celsius scale (International System of Units), Note: The triple point of water is 0.01 °C, not 0 °C; thus 0 K is −2890.15 °C, not −273.16 °C. which equals −459.67 degrees on the Fahrenheit scale ( United States customary units or Imperial units). The corresponding Kelvin and Rankine temperature scales set their zero points at absolute zero by definition. It is commonly thought of as the lowest temperature possible, but it is not the lowest ''enthalpy'' state poss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryogenics
In physics, cryogenics is the production and behaviour of materials at very low temperatures. The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cryogenic” by accepting a threshold of 120 K (or –153 °C) to distinguish these terms from the conventional refrigeration. This is a logical dividing line, since the normal boiling points of the so-called permanent gases (such as helium, hydrogen, neon, nitrogen, oxygen, and normal air) lie below 120K while the Freon refrigerants, hydrocarbons, and other common refrigerants have boiling points above 120K. The U.S. National Institute of Standards and Technology considers the field of cryogenics as that involving temperatures below -153 Celsius (120K; -243.4 Fahrenheit) Discovery of superconducting materials with critical temperatures significantly above the boiling point of nitrogen has provided new interest in reliable, low cost method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Instruments
A thermodynamic instrument is any device which facilitates the quantitative measurement of thermodynamic systems. In order for a thermodynamic parameter to be truly defined, a technique for its measurement must be specified. For example, the ultimate definition of temperature is "what a thermometer reads". The question follows – what is a thermometer? There are two types of thermodynamic instruments, the meter and the reservoir. A thermodynamic meter is any device which measures any parameter of a thermodynamic system. A thermodynamic reservoir is a system which is so large that it does not appreciably alter its state parameters when brought into contact with the test system. Overview Two general complementary tools are the meter and the reservoir. It is important that these two types of instruments are distinct. A meter does not perform its task accurately if it behaves like a reservoir of the state variable it is trying to measure. If, for example, a thermometer, were to act a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boyle's Law
Boyle's law, also referred to as the Boyle–Mariotte law, or Mariotte's law (especially in France), is an experimental gas law that describes the relationship between pressure and volume of a confined gas. Boyle's law has been stated as: The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system.Levine, Ira. N. (1978), p. 12 gives the original definition. Mathematically, Boyle's law can be stated as: or where is the pressure of the gas, is the volume of the gas, and is a constant. Boyle's Law states that when the temperature of a given mass of confined gas is constant, the product of its pressure and volume is also constant. When comparing the same substance under two different sets of conditions, the law can be expressed as: :P_1 V_1 = P_2 V_2. showing that as volume increases, the pressure of a gas decreases proportionally, and vic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combined Gas Law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: pV = nRT where p, V and T are the pressure, volume and temperature; n is the amount of substance; and R is the ideal gas constant. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856 and Rudolf Clausius in 1857. Equation The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the appropriat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gay-Lussac's Law
Gay-Lussac's law usually refers to Joseph-Louis Gay-Lussac's law of combining volumes of gases, discovered in 1808 and published in 1809. It sometimes refers to the proportionality of the volume of a gas to its absolute temperature at constant pressure. This law was published by Gay-Lussac in 1802, and in the article in which he described his work he cited earlier unpublished work from the 1780s by Jacques Charles. Consequently, the volume-temperature proportionality is usually known as Charles's Law. Law of combining volumes The law of combining volumes states that, when gases react together they do so in volume which bears simple whole number ratio provided that the temperature and pressure of the reacting gases and their products remain constant The ratio between the volumes of the reactant gases and the gaseous products can be expressed in simple whole numbers. For example, Gay-Lussac found that two volumes of hydrogen and one volume of oxygen would react to form two vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |