|
Fondant Icing
Fondant icing, also commonly referred to simply as fondant (, from the ), is an icing used to decorate or sculpt cakes and pastries. It is made from sugar, water, gelatin, vegetable fat or shortening, and glycerol. It does not have the texture of most icings; rolled fondant is akin to stiff clay, while poured fondant is a thick liquid. The word, in French, means 'melting,' coming from the same root as '' fondue'' and ''foundry.'' Types of rolled fondant ''Rolled fondant,'' ''fondant icing,'' or ''pettinice,'' which is not the same material as poured fondant, is commonly used to decorate wedding cakes. Although wedding cakes are traditionally made with marzipan and royal icing, fondant is increasingly common due to nut allergies, as it does not require almond meal. Rolled fondant includes gelatin (or agar in vegetarian recipes) and food-grade glycerine, which keeps the sugar pliable and creates a dough-like consistency. Rolled fondant is rolled out like a pie crust and use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
France
France (), officially the French Republic ( ), is a country primarily located in Western Europe. It also comprises of overseas regions and territories in the Americas and the Atlantic, Pacific and Indian Oceans. Its metropolitan area extends from the Rhine to the Atlantic Ocean and from the Mediterranean Sea to the English Channel and the North Sea; overseas territories include French Guiana in South America, Saint Pierre and Miquelon in the North Atlantic, the French West Indies, and many islands in Oceania and the Indian Ocean. Due to its several coastal territories, France has the largest exclusive economic zone in the world. France borders Belgium, Luxembourg, Germany, Switzerland, Monaco, Italy, Andorra, and Spain in continental Europe, as well as the Netherlands, Suriname, and Brazil in the Americas via its overseas territories in French Guiana and Saint Martin. Its eighteen integral regions (five of which are overseas) span a combined area of and contain clos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
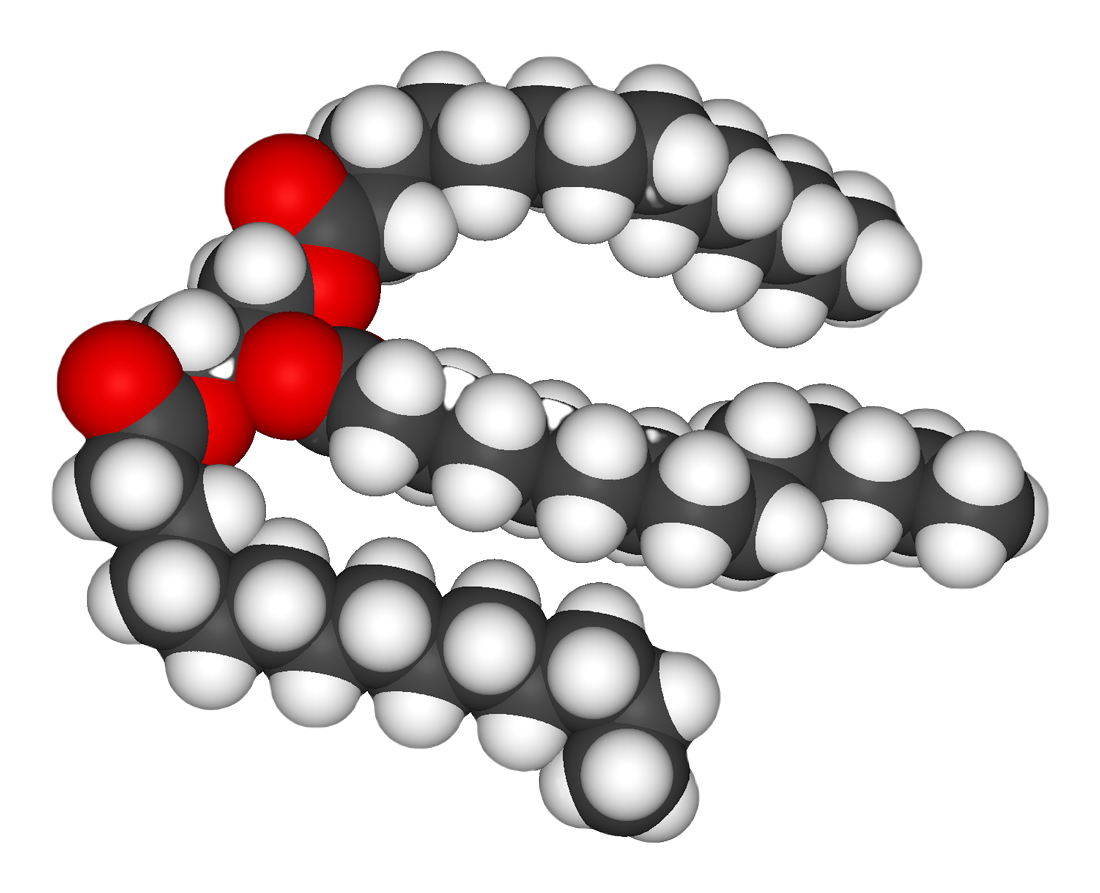
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. The majority of minerals and organic molecules crystallize easily, and the resulting crystals are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seed Crystal
A seed crystal is a small piece of single crystal or polycrystal material from which a large crystal of typically the same material is grown in a laboratory. Used to replicate material, the use of seed crystal to promote growth avoids the otherwise slow randomness of natural crystal growth and allows manufacture on a scale suitable for industry. Crystal enlargement The large crystal can be grown by dipping the seed into a supersaturated solution, into molten material that is then cooled, or by growth on the seed face by passing vapor of the material to be grown over it. Theory The theory behind this effect is thought to derive from the physical intermolecular interaction that occurs between compounds in a supersaturated solution (or possibly vapor). In solution, liberated (soluble) molecules ( solute) are free to move about in random flow. This random flow permits for the possibility of two or more molecular compounds to interact. This interaction can potentiate intermolecula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) below 0°C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods ( supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phases at continuous phase transitions start to form imm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. A liquid at low pressure has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmosphe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula . For human consumption, sucrose is extracted and refined from either sugarcane or sugar beet. Sugar mills – typically located in tropical regions near where sugarcane is grown – crush the cane and produce raw sugar which is shipped to other factories for refining into pure sucrose. Sugar beet factories are located in temperate climates where the beet is grown, and process the beets directly into refined sugar. The sugar-refining process involves washing the raw sugar crystals before dissolving them into a sugar syrup which is filtered and then passed over carbon to remove any residual colour. The sugar syrup is then concentrated by boiling under a vacuum and crystallized as the final purification process to produce crystals of pure sucrose that are clear, odorless, and sweet. Su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supersaturation
In physical chemistry, supersaturation occurs with a solution (chemistry), solution when the concentration of a solute exceeds the concentration specified by the value of solubility at Solubility equilibrium, equilibrium. Most commonly the term is applied to a solution of a solid in a liquid. A supersaturated solution is in a metastable state; it may be brought to equilibrium by forcing the excess of solute to separation process, separate from the solution. The term can also be applied to a mixture of gases. History Early studies of the phenomenon were conducted with sodium sulfate, also known as Glauber's Salt because, unusually, the solubility of this salt in water may decrease with increasing temperature. Early studies have been summarised by Tomlinson. It was shown that the crystallization of a supersaturated solution does not simply come from its agitation, (the previous belief) but from solid matter entering and acting as a "starting" site for crystals to form, now calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxymethyl Cellulose
Carboxymethyl cellulose (CMC) or cellulose gum is a cellulose derivative with carboxymethyl groups (-CH2-COOH) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is often used as its sodium salt, sodium carboxymethyl cellulose. It used to be marketed under the name Tylose, a registered trademark of SE Tylose. Preparation Carboxymethyl cellulose is synthesized by the alkali-catalyzed reaction of cellulose with chloroacetic acid. The polar (organic acid) carboxyl groups render the cellulose soluble and chemically reactive. Fabrics made of cellulose—e.g. cotton or viscose rayon—may also be converted into CMC. Following the initial reaction, the resultant mixture produces approximately 60% CMC and 40% salts ( sodium chloride and sodium glycolate); this product is the so-called technical CMC, which is used in detergents. An additional purification process is used to remove salts to produce pure CMC, which is used for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shortening
Shortening is any fat that is a solid at room temperature and used to make crumbly pastry and other food products. Although butter is solid at room temperature and is frequently used in making pastry, the term ''shortening'' seldom refers to butter. The idea of shortening dates back to at least the 18th century, well before the invention of modern, shelf-stable vegetable shortening. In the earlier centuries, lard was the primary ingredient used to shorten dough. The reason it is called shortening is that it makes the resulting food crumbly, or to behave as if it has short fibers. Solid fat prevents cross-linkage between gluten molecules. This cross-linking would give dough elasticity, so it could be stretched into longer pieces. In pastries such as cake, which should not be elastic, shortening is used to produce the desired texture. History and market Originally shortening was synonymous with lard, but with the invention of margarine from beef tallow by French chemist H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugar Paste
Sugar paste icing is a sweet, edible sugar dough, typically made from sucrose and glucose. It is sometimes referred to as sugar gum or gum paste. Though the two are both used in cake decorating, sugar paste differs from fondant icing Fondant icing, also commonly referred to simply as fondant (, from the ), is an icing used to decorate or sculpt cakes and pastries. It is made from sugar, water, gelatin, vegetable fat or shortening, and glycerol. It does not have the textur ... in that it hardens, rather than retaining a soft consistency, making it ideal for creating solid, sculpted decorations that can later be attached to a cake by other means. By contrast, the soft and malleable qualities of fondant icing make it softer and more ideal for covering cakes entirely. Production Sugar paste is produced both commercially and domestically, with commercial sugar paste holding a number of advantages that homemade sugar paste does not; commercial varieties of sugar paste can be s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marshmallow
Marshmallow (, ) is a type of confectionery that is typically made from sugar, water and gelatin whipped to a solid-but-soft consistency. It is used as a filling in baking or normally molded into shapes and coated with corn starch. The sugar confection is inspired by a historical medicinal confection made from '' Althaea officinalis'', the marsh-mallow plant. History The word "marshmallow" comes from the mallow plant species ('' Althaea officinalis''), a herb native to parts of Europe, North Africa, and Asia which grows in marshes and other damp areas. The plant's stem and leaves are fleshy, and its white flower has five petals. It is not known exactly when marshmallows were invented, but their history goes back as early as . Ancient Egyptians were said to be the first to make and use the root of the plant to soothe coughs and sore throats and to heal wounds. The first marshmallows were prepared by boiling pieces of root pulp with honey until thick. Once thickened, the mixt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |