|
Focal Adhesion
In cell biology, focal adhesions (also cell–matrix adhesions or FAs) are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects (i.e., signaling events) of a cell in response to ECM adhesion. Focal adhesions serve as the mechanical linkages to the ECM, and as a biochemical signaling hub to concentrate and direct numerous signaling proteins at sites of integrin binding and clustering. Structure and function Focal adhesions are integrin-containing, multi-protein structures that form mechanical links between intracellular actin bundles and the extracellular substrate in many cell types. Focal adhesions are large, dynamic protein complexes through which the cytoskeleton of a cell connects to the ECM. They are limited to clearly defined ranges of the cell, at which the pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
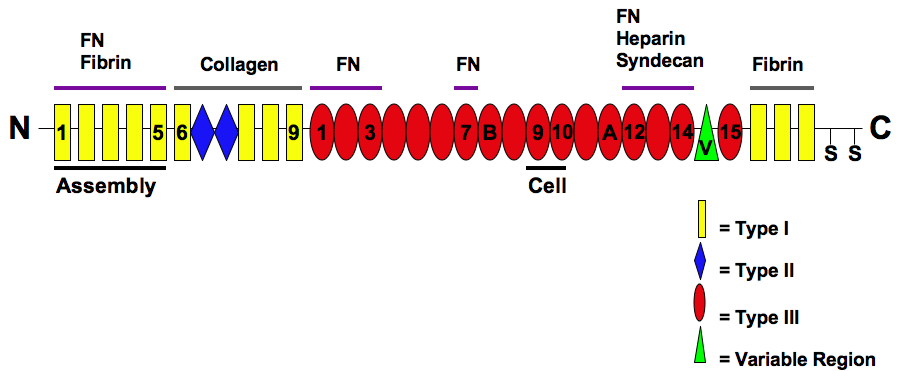
Fibronectin
Fibronectin is a high- molecular weight (~500-~600 kDa) glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans (e.g. syndecans). Fibronectin exists as a protein dimer, consisting of two nearly identical monomers linked by a pair of disulfide bonds. The fibronectin protein is produced from a single gene, but alternative splicing of its pre-mRNA leads to the creation of several isoforms. Two types of fibronectin are present in vertebrates: * soluble plasma fibronectin (formerly called "cold-insoluble globulin", or CIg) is a major protein component of blood plasma (300 μg/ml) and is produced in the liver by hepatocytes. * insoluble cellular fibronectin is a major component of the extracellular matrix. It is secreted by various cells, primarily fibroblasts, as a soluble protein dimer and is then ass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Talin Protein
Talin is a high-molecular-weight cytoskeletal protein concentrated at regions of cell–substratum contact and, in lymphocytes, at cell–cell contacts. Discovered in 1983 by Keith Burridge and colleagues, talin is a ubiquitous cytosolic protein that is found in high concentrations in focal adhesions. It is capable of linking integrins to the actin cytoskeleton either directly or indirectly by interacting with vinculin and α-actinin. Also, talin-1 drives extravasation mechanism through engineered human microvasculature in microfluidic systems. Talin-1 is involved in each part of extravasation affecting adhesion, trans-endothelial migration and the invasion stages. Integrin receptors are involved in the attachment of adherent cells to the extracellular matrix and of lymphocytes to other cells. In these situations, talin codistributes with concentrations of integrins in the plasma membrane. Furthermore, in vitro binding studies suggest that integrins bind to talin, although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
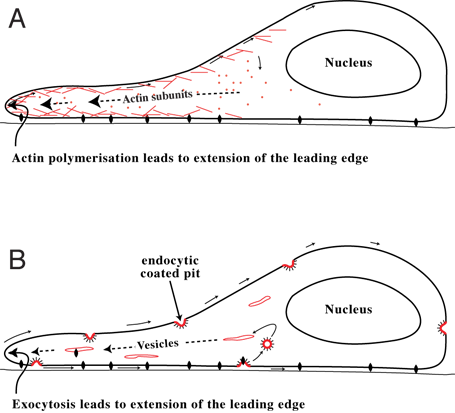
Lamellipodia
The lamellipodium (plural lamellipodia) (from Latin ''lamella'', related to ', "thin sheet", and the Greek radical ''pod-'', "foot") is a cytoskeletal protein actin projection on the leading edge of the cell. It contains a quasi-two-dimensional actin mesh; the whole structure propels the cell across a substrate. Within the lamellipodia are ribs of actin called microspikes, which, when they spread beyond the lamellipodium frontier, are called filopodia. The lamellipodium is born of actin nucleation in the plasma membrane of the cell and is the primary area of actin incorporation or microfilament formation of the cell. Description Lamellipodia are found primarily in all mobile cells, such as the keratinocytes of fish and frogs, which are involved in the quick repair of wounds. The lamellipodia of these keratinocytes allow them to move at speeds of 10–20 μm / min over epithelial surfaces. When separated from the main part of a cell, a lamellipodium can still c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
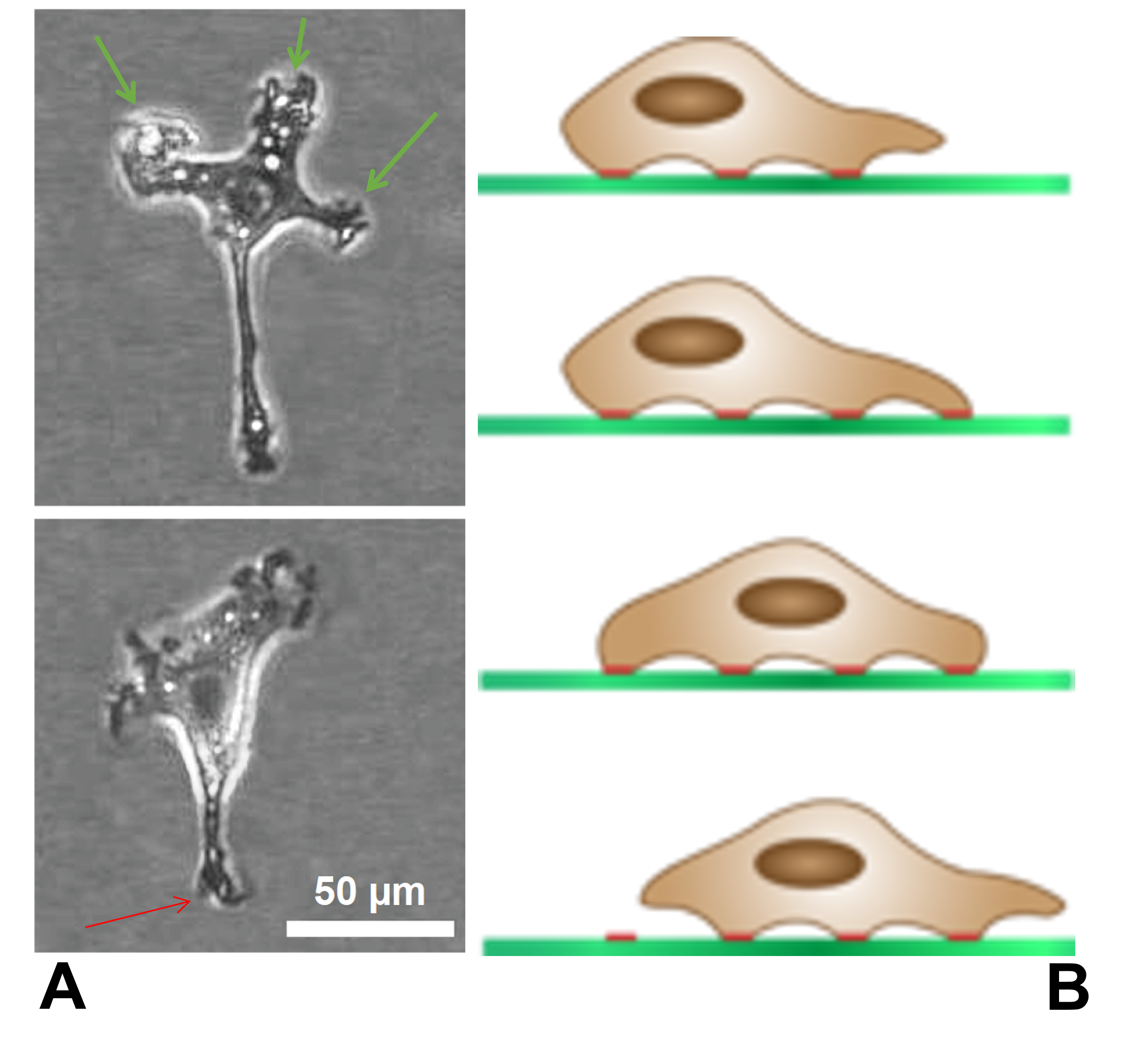
Cell Migration
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryonic development, wound healing and immune responses all require the orchestrated movement of cells in particular directions to specific locations. Cells often migrate in response to specific external signals, including chemical signals and mechanical signals. Errors during this process have serious consequences, including intellectual disability, vascular disease, tumor formation and metastasis. An understanding of the mechanism by which cells migrate may lead to the development of novel therapeutic strategies for controlling, for example, invasive tumour cells. Due to the highly viscous environment (low Reynolds number), cells need to continuously produce forces in order to move. Cells achieve active movement by very different mechanisms. Many less complex prokaryotic organisms (and sperm cells) use flagella or cilia to propel themselves. Eukaryot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Focal Adhesion Kinase
PTK2 protein tyrosine kinase 2 (PTK2), also known as focal adhesion kinase (FAK), is a protein that, in humans, is encoded by the ''PTK2'' gene. PTK2 is a focal adhesion-associated protein kinase involved in cellular adhesion (how cells stick to each other and their surroundings) and spreading processes (how cells move around). It has been shown that when FAK was blocked, breast cancer cells became less metastatic due to decreased mobility. Function The PTK2 gene encodes a cytosolic protein tyrosine kinase that is found concentrated in the focal adhesions that form among cells attaching to extracellular matrix constituents. The encoded protein is a member of the FAK subfamily of protein tyrosine kinases that included PYK2, but lacks significant sequence similarity to kinases from other subfamilies. It also includes a large FERM domain. With the exception of certain types of blood cells, most cells express FAK. FAK tyrosine kinase activity can be activated, which plays a key ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tensin
Tensin was first identified as a 220 kDa multi-domain protein localized to the specialized regions of plasma membrane called integrin-mediated focal adhesions (which are formed around a transmembrane core of an αβ integrin heterodimer). Genome sequencing and comparison have revealed the existence of four tensin genes in humans. These genes appear to be related by ancient instances of gene duplication. Tensin binds to actin filaments and contains a phosphotyrosine-binding (PTB) domain at the C-terminus, which interacts with the cytoplasmic tail of β integrins. These interactions allow tensin to link actin filaments to integrin receptors. Several factors induce tyrosine phosphorylation of tensin. Thus, tensin functions as a platform for assembly and disassembly of signaling complexes at focal adhesions by recruiting tyrosine-phosphorylated signaling molecules, and also by providing interaction sites for other proteins. Haynie, by contrast, argues in a review of tensin structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinculin
In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane. Discovered independently by Benny Geiger and Keith Burridge, its sequence is 20%–30% similar to α-catenin, which serves a similar function. Binding alternately to talin or α-actinin, vinculin's shape and, as a consequence, its binding properties are changed. The vinculin gene occurs as a single copy and what appears to be no close relative to take over functions in its absence. Its splice variant metavinculin (see below) also needs vinculin to heterodimerize and work in a dependent fashion. Structure Vinculin is a 117-kDa cytoskeletal protein with 1066 amino acids. The protein contains an acidi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filamin
Filamins are a class of proteins that hold two actin filaments at large angles. Filamin protein in mammals is made up of an actin-binding domain at its N-terminus that is followed by 24 immunoglobulin-like repeat modules of roughly 95 amino acids. There are two hinge regions; between repeats 15-16 and 23-24. Filamin gets cleaved at these hinge regions to generate smaller fragments of the protein. Filamin has two actin-binding sites with a V-linkage between them, so that it cross-links actin filaments into a network with the filaments orientated almost at right angles to one another. Filamin proteins include: * FLNA * FLNB * FLNC Over-expression of FLNA stops the regeneration of bladder carcinoma (BC) cells, by inhibiting the cell cycle and inducing apoptosis of BC cells. FLNA has also been shown to reduce the mobility Mobility may refer to: Social sciences and humanities * Economic mobility, ability of individuals or families to improve their economic status * Geographic mobi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinin, Alpha 1
Alpha-actinin-1 is a protein that in humans is encoded by the ''ACTN1'' gene. Function Alpha actinins belong to the spectrin gene superfamily which represents a diverse group of cytoskeletal proteins, including the alpha and beta spectrins and dystrophins. Alpha-actinin-1 is an F-actin cross-linking protein – a bundling protein that is thought to anchor actin to a number of intracellular structures. Alpha-actinin-1 is a non-muscle cytoskeletal isoform found along microfilament bundles and adherens-type junctions, where it is involved in binding actin to the membrane. In contrast, skeletal, cardiac, and smooth muscle isoforms are localized to the Z-disc and analogous dense bodies, where they help anchor the myofibrillar actin filaments. Interactions Alpha-actinin-1 has been shown to interact with: * CDK5R1, * CDK5R2, * Collagen, type XVII, alpha 1, * GIPC1, * PDLIM1, * Protein kinase N1, * SSX2IP, and * Zyxin. *PTPRT (PTPrho) See also * Actinin Actinin is a microfilame ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Talin (protein)
Talin is a high-molecular-weight cytoskeletal protein concentrated at regions of cell–substratum contact and, in lymphocytes, at cell–cell contacts. Discovered in 1983 by Keith Burridge and colleagues, talin is a ubiquitous cytosolic protein that is found in high concentrations in focal adhesions. It is capable of linking integrins to the actin cytoskeleton either directly or indirectly by interacting with vinculin and α-actinin. Also, talin-1 drives extravasation mechanism through engineered human microvasculature in microfluidic systems. Talin-1 is involved in each part of extravasation affecting adhesion, trans-endothelial migration and the invasion stages. Integrin receptors are involved in the attachment of adherent cells to the extracellular matrix and of lymphocytes to other cells. In these situations, talin codistributes with concentrations of integrins in the plasma membrane. Furthermore, in vitro binding studies suggest that integrins bind to talin, although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterodimers
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + ''-mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |