|
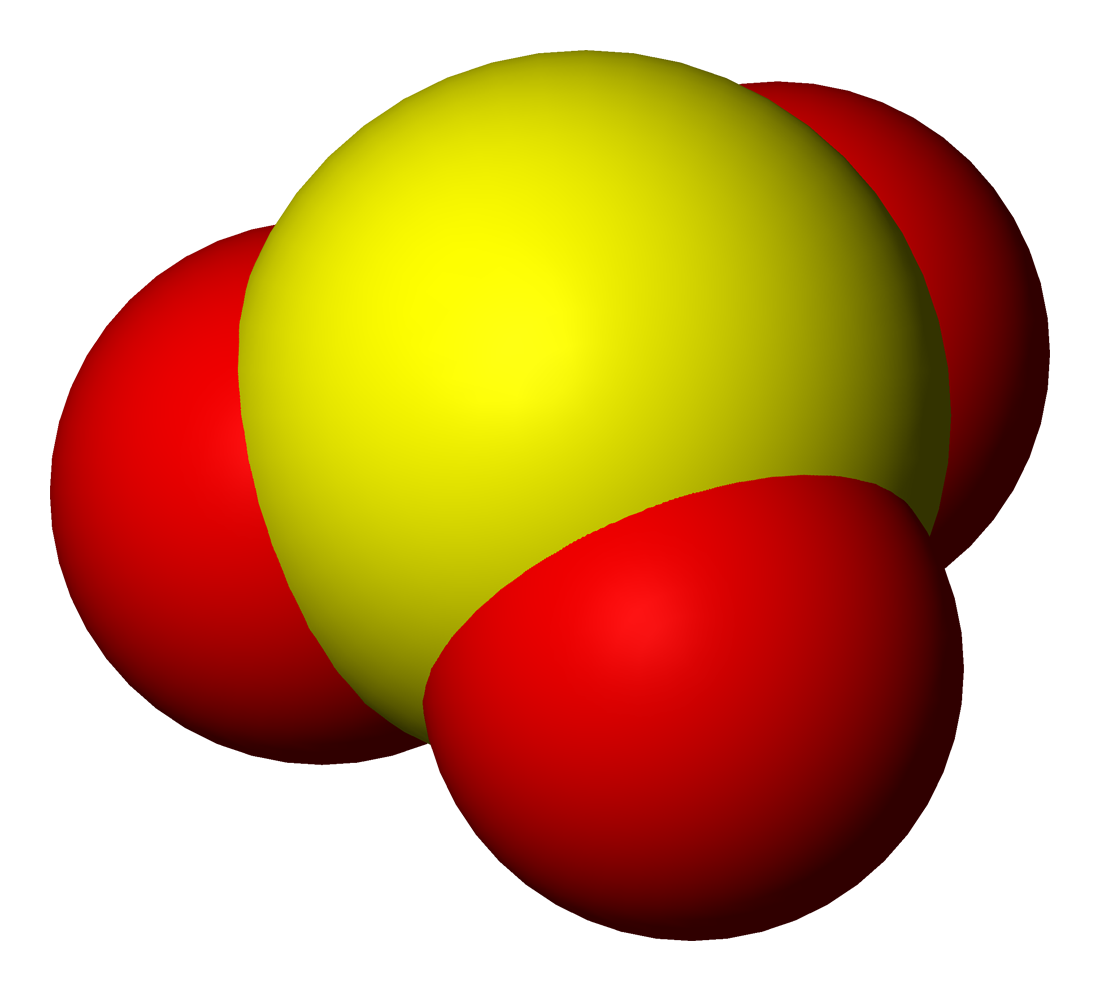
Fluorosulfite
Fluorosulfite is an ion with the formula SO2F−. The term is also used for compounds or salts containing this group. Fluorosulfite was discovered in 1953 by F Seel and H Meier. Organic compounds with the name "fluorosulfite" contain the group -OS(O)F. Preparation (CH3)2N)3SOSO2F] can be prepared from Thionyl tetrafluoride, OSF4 and Me3SiNMe2. Alkali metal fluorosulfites can be made by soaking the metal fluoride in liquid sulfur dioxide for a few days. β-CsSO2F converts to α-CsSO2F when heated to 110 °C for a couple of days but remains stable below 50 °C. Properties The fluorosulfite ion is tetrahedral, with sulfur at the top. The oxygen to sulfur bonds are 147.8 pm and the fluorine to sulfur bond is >169.0 pm long. In solid ionic fluorosulfites, the ion is not fixed in orientation and continuously turns around resulting in dynamic disorder. At room temperature this turning rate is from 2×105 to 107 Hz. When cooled the rate of rotation slows, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thionyl Tetrafluoride
Thionyl tetrafluoride is an inorganic compound gas with the formula S O F4. It is also known as sulfur tetrafluoride oxide. The shape of the molecule is a distorted trigonal bipyramid, with the oxygen found on the equator. The atoms on the equator have shorter bond lengths than the fluorine atoms on the axis. The sulfur oxygen bond is 1.409Å. A S−F bond on the axis has length 1.596Å and the S−F bond on the equator has length 1.539Å. The angle between the equatorial fluorine atoms is 112.8°. The angle between axial fluorine and oxygen is 97.7°. The angle between oxygen and equatorial fluorine is 123.6° and between axial and equatorial fluorine is 85.7°. The fluorine atoms only produce one NMR line, probably because they exchange positions. Formation Thionyl fluoride reacting with fluorine gas can produce thionyl tetrafluoride.Harry Julius Emeléus and A.G. Sharpe ''Advances in Inorganic Chemistry Volume 2'' Academic Press 1960 page 11/ref> This was how the g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the production o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picometre
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer (American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth of a metre, which is the SI base unit of length. The picometre is one thousand femtometres, one thousandth of a nanometre ( nm), one millionth of a micrometre (also known as a micron), one billionth of a millimetre, and one trillionth of a metre. The symbol μμ was once used for it. It is also one hundredth of an ångström, an internationally known (but non-SI) unit of length. Use The picometre's length is of an order so small that its application is almost entirely confined to particle physics, quantum physics, chemistry and acoustics. Atoms are between 62 and 520 pm in diameter, and the typical length of a carbon–carbon single bond is 154 pm. Smaller units still may be used to describe smaller particles (some of which are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoelectronicity
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the structure. For example, , , and are isoelectronic, while and = are not. This definition is sometimes termed ''valence isoelectronicity''. Definitions can sometimes be not as strict, sometimes requiring identity of the ''total'' electron count and with it the entire electronic configuration. More usually, definitions are broader, and may extend to allowing different numbers of atoms in the species being compared.A. A. Aradi & T. P. Fehlner, "Isoelectronic Organometallic Molecules", in F. G. A. Stone & Robert West (eds.) ''Advances in Organometallic Chemistry Vol. 30'' (1990), Chapter 5 (at p. 190google books link/ref> The importance of the concept lies in identifying significantly related species, as pairs or series. Isoelectronic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloryl Fluoride
Chloryl fluoride is the chemical compound with the formula ClO2F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources. It is the acyl fluoride of chloric acid. Preparation ClO2F was first reported by Schmitz and Schumacher in 1942, who prepared it by the fluorination of ClO2. The compound is more conveniently prepared by treatment of sodium chlorate and chlorine trifluoride and purified by vacuum fractionation, i.e. selectively condensing this species separately from other products. This species is a gas boiling at −6 °C: :6 NaClO3 + 4 ClF3 → 6 ClO2F + 2 Cl2 + 3 O2 + 6 NaF Structure In contrast to O2F2, ClO2F is a pyramidal molecule. This structure is predicted by VSEPR. The differing structures reflects the greater tendency of chlorine to exist in positive oxidation states with oxygen and fluorine ligands. The related Cl-O-F compound perchloryl fluoride Perchloryl fluoride is a reactive gas with the chemical formula . It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine. As predicted by valence shell electron pair repulsion theory, chlorate anions have trigonal pyramidal structures. Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials. Mixtures of chlorate salts with virtually any combustible material (sugar, sawdust, charcoal, organic solvents, metals, etc.) will readily deflagrate. Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability. Most pyrotechnic applications that formerly used chlorates now use the more stable perchlorates instead. Structure and bonding The chlorate ion cannot be satisf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfites
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |