|
Sulfites
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Bisulfite
Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. For properties of sodium bisulfite, refer to the table located to the right. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry. Synthesis Sodium bisulfite solutions can be prepared by treating a solution of suitable base, such as sodium hydroxide or sodium bicarbonate with sulfur dioxide. :SO2 + NaOH → NaHSO3 :SO2 + NaHCO3 → NaHSO3 + CO2 Attempts to crystallize the product yield So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
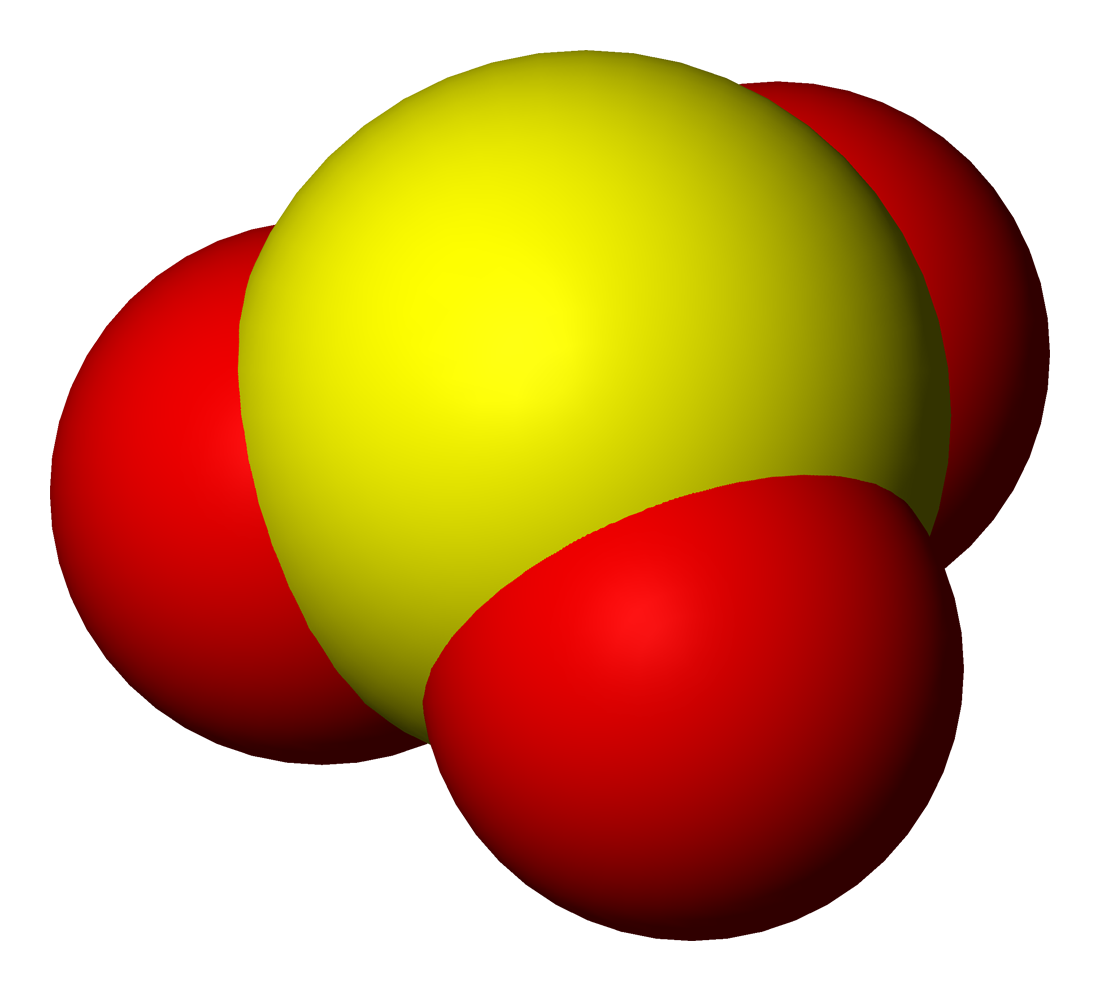
Sulfur Dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur- bearing fossil fuels. Structure and bonding SO2 is a bent molecule with ''C''2v symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke ''d'' orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. Occurrence Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. On other p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur- bearing fossil fuels. Structure and bonding SO2 is a bent molecule with ''C''2v symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke ''d'' orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. Occurrence Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. On other p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Metabisulfite
Potassium metabisulfite, K2S2O5, also known as potassium pyrosulfite, is a white crystalline powder with a pungent odour. It is mainly used as an antioxidant or chemical sterilant. As a disulfite, it is chemically very similar to sodium metabisulfite, with which it is sometimes used interchangeably. Potassium metabisulfite has a monoclinic crystal structure. Preparation and reactions Potassium metabisulfite can be prepared by treating a solution of potassium hydroxide with sulfur dioxide. :2 SO2 + 2 KOH → K2S2O5 + H2O It decomposes at 190 °C, yielding potassium sulfite and sulfur dioxide: :K2S2O5 → K2SO3 + SO2 Uses It is used as a food additive, also known as E224. It is restricted in use and may cause allergic reactions in some sensitive persons. Wine Potassium metabisulfite is a common wine or must additive, in which it forms sulfur dioxide (SO2). Sulfur dioxide is a disinfectant. It also acts as a potent antioxidant, protecting both the color and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Bisulfite
Potassium bisulfite (or potassium hydrogen sulfite) is a chemical mixture with the approximate chemical formula KHSO3. Potassium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of potassium ions and bisulfite ions. It is a white solid with an odor of sulfur dioxide. Attempts to crystallize potassium bisulfite yield potassium metabisulfite, K2S2O5. Potassium bisulfite is used as a sterilising agent in the production of alcoholic beverages. This additive is classified as E number E228 under the current EU-approved food additive legislation. Production It is made by the reaction of sulfur dioxide and potassium carbonate. The sulfur dioxide is passed through a solution of the potassium carbonate until no more carbon dioxide is evolved. The solution is concentrated. See also * Calcium bisulfite * Sodium bisulfite Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisulfite
The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion . Salts containing the ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+. :Na2S2O5 + H2O → 2Na SO3 Structure The bisulfite anion exists in solution as a mixture of two tautomers. One tautomer has the proton attached to one of the three oxygen centers. In the second tautomer the proton resides on sulfur. The S-protonated tautomer has ''C''3v symmetry. The O-protonated tautomer has only Cs symmetry. Reactions Tautomerization There exist two tautomers of bisulfite. They interconvert readily but can be characterized individually by various spectroscopic methods. They have been observed by 17O NMR spectroscopy: :HSO3− SO2(OH)− K = 4.2 Acid-base reactions Solutions of bisulfite are typically prepared by treatment of sulfur dioxide with aqueous base: :S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Metabisulfite
Sodium metabisulfite or sodium pyrosulfite (IUPAC spelling; Br. E. sodium metabisulphite or sodium pyrosulphite) is an inorganic compound of chemical formula Na2S2O5. The substance is sometimes referred to as disodium metabisulfite. It is used as a disinfectant, antioxidant, and preservative agent. Preparation Sodium metabisulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide. When conducted in warm water, Na2SO3 initially precipitates as a yellow solid. With more SO2, the solid dissolves to give the disulfite, which crystallises upon cooling. :SO2 + 2 NaOH → Na2SO3 + H2O :SO2 + Na2SO3 → Na2S2O5 which yields a residue of colourless solid Na2S2O5. Chemical structure The anion metabisulfite consists of an SO2 group linked to an SO3 group, with the negative charge more localised on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å, respectively. Reactivity Upon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Sulfite
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2 SO3. A white, water-soluble solid, it is used commercially as an antioxidant and preservative. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air. Preparation Sodium sulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide. When conducted in warm water, Na2SO3 initially precipitates as a white solid. With more SO2, the solid dissolves to give the disulfite, which crystallizes upon cooling. :SO2 + 2 NaOH -> Na2SO3 + H2O Sodium sulfite is made industrially by treating sulfur dioxide with a solution of sodium carbonate. The overall reaction is: :SO2 + Na2CO3 -> Na2SO3 + CO2 Applications Sodium sulfite is primarily used in the pulp and paper industry. It has been also applied in the thermomechanical conversion of wood to fibres (''defibration'') for producing medium density fibreboard ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfurous Acid
Sulfurous acid (also sulfuric(IV) acid, sulphurous acid (UK), sulphuric(IV) acid (UK)) is the chemical compound with the formula . There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase. The conjugate bases of this elusive acid are, however, common anions, bisulfite (or hydrogen sulfite) and sulfite. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide. Raman spectra of solutions of sulfur dioxide in water show only signals due to the molecule and the bisulfite ion, . The intensities of the signals are consistent with the following equilibrium: 17O NMR spectroscopy provided evidence that solutions of sulfurous acid and protonated sulfites contain a mixture of isomers, which is in equilibrium: Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxde and water vapor. A clathrate with the formul a has been crystallised ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wine
Wine is an alcoholic drink typically made from fermented grapes. Yeast consumes the sugar in the grapes and converts it to ethanol and carbon dioxide, releasing heat in the process. Different varieties of grapes and strains of yeasts are major factors in different styles of wine. These differences result from the complex interactions between the biochemical development of the grape, the reactions involved in fermentation, the grape's growing environment (terroir), and the wine production process. Many countries enact legal appellations intended to define styles and qualities of wine. These typically restrict the geographical origin and permitted varieties of grapes, as well as other aspects of wine production. Wines not made from grapes involve fermentation of other crops including rice wine and other fruit wines such as plum, cherry, pomegranate, currant and elderberry. Wine has been produced for thousands of years. The earliest evidence of wine is from the Caucasus reg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermentation (wine)
The process of fermentation in winemaking turns grape juice into an alcoholic beverage. During fermentation, yeasts transform sugars present in the juice into ethanol and carbon dioxide (as a by-product). In winemaking, the temperature and speed of fermentation are important considerations as well as the levels of oxygen present in the must at the start of the fermentation. The risk of stuck fermentation and the development of several wine faults can also occur during this stage, which can last anywhere from 5 to 14 days for ''primary fermentation'' and potentially another 5 to 10 days for a '' secondary fermentation''. Fermentation may be done in stainless steel tanks, which is common with many white wines like Riesling, in an open wooden vat, inside a wine barrel and inside the wine bottle itself as in the production of many sparkling wines. History The natural occurrence of fermentation means it was probably first observed long ago by humans.H. Johnson: ''Vintage: The Sto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |