|
FeMoco
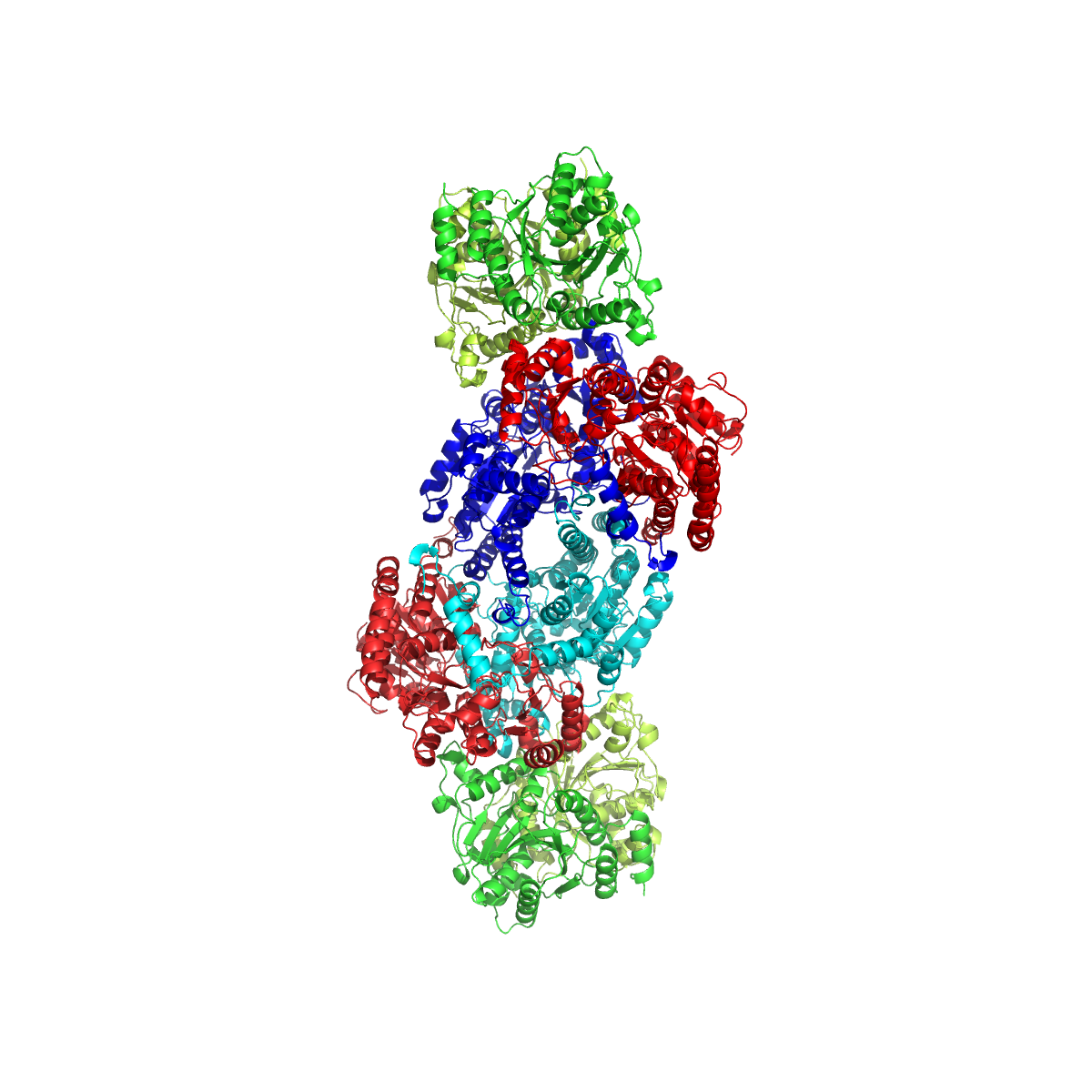
FeMoco ( cofactor) is the primary cofactor of nitrogenase. Nitrogenase is the enzyme that catalyzes the conversion of atmospheric nitrogen molecules N2 into ammonia (NH3) through the process known as nitrogen fixation. Containing iron and molybdenum, the cofactor is called FeMoco. Its stoichiometry is Fe7MoS9C. Structure The FeMo cofactor is a cluster with composition Fe7MoS9C. Fe is the chemical symbol for the element iron (ferrum), and Mo is the symbol for molybdenum. This large cluster can be viewed as two subunits composed of one Fe4S3 ( iron(III) sulfide) cluster and one MoFe3S3 cluster. The two clusters are linked by three sulfide ligands. The unique iron (Fe) is anchored to the protein by a cysteine. It is also bound to three sulfides, resulting in tetrahedral molecular geometry. The additional six Fe centers in the cluster are each bonded to three sulfides. These six internal Fe centers define a trigonal prismatic arrangement around a central carbide center. The mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FeMoco Cluster
FeMoco ( cofactor) is the primary cofactor of nitrogenase. Nitrogenase is the enzyme that catalyzes the conversion of atmospheric nitrogen molecules N2 into ammonia (NH3) through the process known as nitrogen fixation. Containing iron and molybdenum, the cofactor is called FeMoco. Its stoichiometry is Fe7MoS9C. Structure The FeMo cofactor is a cluster with composition Fe7MoS9C. Fe is the chemical symbol for the element iron (ferrum), and Mo is the symbol for molybdenum. This large cluster can be viewed as two subunits composed of one Fe4S3 (iron(III) sulfide) cluster and one MoFe3S3 cluster. The two clusters are linked by three sulfide ligands. The unique iron (Fe) is anchored to the protein by a cysteine. It is also bound to three sulfides, resulting in tetrahedral molecular geometry. The additional six Fe centers in the cluster are each bonded to three sulfides. These six internal Fe centers define a trigonal prismatic arrangement around a central carbide center. The molyb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum(IV) Compounds
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a free metal on Earth; it is found only in various oxidation states in minerals. The free element, a silvery metal with a grey cast, has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum compounds have low solubility ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a free metal on Earth; it is found only in various oxidation states in minerals. The free element, a silvery metal with a grey cast, has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum compounds have low so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a key step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules ( nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase. Classification and structure Although the equilibrium formation of ammonia from molecular hydrogen and nitrogen has an overall negative enthalpy of reaction ( \Delta H^ = -45.2 \ \mathrm \, \mathrm \; \mathrm ), the activation energy is very high ( E_\mathrm = 230-420 \ \mathrm \, \mathrm ). Nitrogenase acts as a catalyst, reducing this energy barr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmospheric nitrogen is molecular dinitrogen, a relatively nonreactive molecule that is metabolically useless to all but a few microorganisms. Biological nitrogen fixation or ''diazotrophy'' is an important microbials mediated process that converts dinitrogen (N2) gas to ammonia (NH3) using the nitrogenase protein complex (Nif). Nitrogen fixation is essential to life because fixed inorganic nitrogen compounds are required for the biosynthesis of all nitrogen-containing organic compounds, such as amino acids and proteins, nucleoside triphosphates and nucleic acids. As part of the nitrogen cycle, it is essential for agriculture and the manufacture of fertilizer. It is also, indirectly, relevant to the manufacture of all nitrogen chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Methylformamide
''N''-Methylformamide (NMF) is a colorless, nearly odorless, organic compound and secondary amide with molecular formula CH3NHCHO, which is a liquid at room temperature. NMF is mainly used as a reagent in various organic syntheses with limited applications as a highly polar solvent. NMF is closely related to other formamides, notably formamide and dimethylformamide (DMF). However, industrial use and production of NMF are far less than for either of these other formamides. DMF is favored over NMF as a solvent due to its greater stability. Annual production of NMF can be assumed to be significantly less than the production of either formamide (100,000 tons) or DMF (500,000 tons). Structure and properties Like DMF and formamide, each of the two rotamers of NMF are described by two principal resonance structures: :: This description highlights the partial double bond that exists between the carbonyl carbon and nitrogen, which gives rise to a high rotational barrier. Thus, the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis
Biosynthesis is a multi-step, enzyme- catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism. The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g. NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nif Gene
The ''nif'' genes are genes encoding enzymes involved in the fixation of atmospheric nitrogen into a form of nitrogen available to living organisms. The primary enzyme encoded by the ''nif'' genes is the nitrogenase complex which is in charge of converting atmospheric nitrogen (N2) to other nitrogen forms such as ammonia which the organism can use for various purposes. Besides the nitrogenase enzyme, the ''nif'' genes also encode a number of regulatory proteins involved in nitrogen fixation. The ''nif'' genes are found in both free-living nitrogen-fixing bacteria and in symbiotic bacteria associated with various plants. The expression of the ''nif'' genes is induced as a response to low concentrations of fixed nitrogen and oxygen concentrations (the low oxygen concentrations are actively maintained in the root environment of host plants). The first Rhizobium genes for nitrogen fixation (nif) and for nodulation (nod) were cloned in the early 1980s by Gary Ruvkun and Sharon R. Long ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homocitrate Synthase
In enzymology, a homocitrate synthase () is an enzyme that catalyzes the chemical reaction :acetyl-CoA + H2O + 2-oxoglutarate \rightleftharpoons (R)-2-hydroxybutane-1,2,4-tricarboxylate + CoA The 3 substrates of this enzyme are acetyl-CoA, H2O, and 2-oxoglutarate, whereas its two products are (R)-2-hydroxybutane-1,2,4-tricarboxylate and CoA. This enzyme belongs to the family of transferases, specifically those acyltransferases that convert acyl groups into alkyl groups on transfer. The systematic name of this enzyme class is acetyl-CoA:2-oxoglutarate C-acetyltransferase (thioester-hydrolysing, carboxymethyl forming). Other names in common use include 2-hydroxybutane-1,2,4-tricarboxylate 2-oxoglutarate-lyase, (CoA-acetylating), acetyl-coenzyme A:2-ketoglutarate C-acetyl transferase, and homocitrate synthetase. This enzyme participates in lysine biosynthesis and pyruvate metabolism Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N,N-dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless, but technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by sparging samples with an inert gas such as argon or by sonicating the samples under reduced pressure. As its name indicates, it is structurally related to formamide, having two methyl groups in the place of the two hydrogens. DMF is a polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such as SN2 reactions. Structure and properties As for most amides, the spectroscopic evidence indicates partial double bond chara ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Compounds
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron Age. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffractometry
A diffractometer is a measuring instrument for analyzing the structure of a material from the scattering pattern produced when a beam of radiation or particles (such as X-rays or neutrons) interacts with it. Principle Because it is relatively easy to use electrons or neutrons having wavelengths smaller than a nanometer, electrons and neutrons may be used to study crystal structure in a manner very similar to X-ray diffraction. Electrons do not penetrate as deeply into matter as X-rays, hence electron diffraction reveals structure near the surface; neutrons do penetrate easily and have an advantage that they possess an intrinsic magnetic moment that causes them to interact differently with atoms having different alignments of their magnetic moments. A typical diffractometer consists of a source of radiation, a monochromator to choose the wavelength, slits to adjust the shape of the beam, a sample and a detector. In a more complicated apparatus, a goniometer can also be used for fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |