|
Molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a free metal on Earth; it is found only in various oxidation states in minerals. The free element, a silvery metal with a grey cast, has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum compounds have low so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
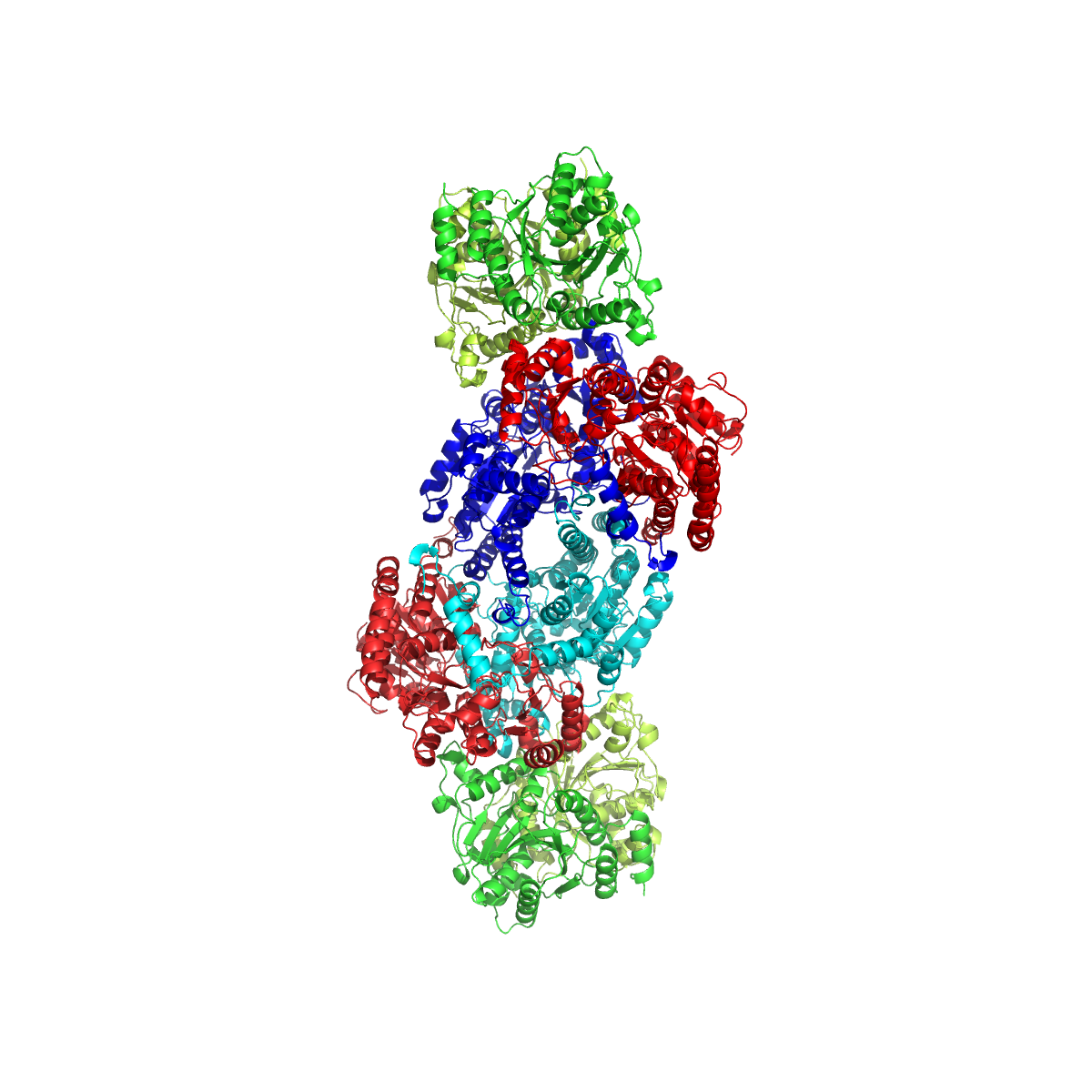
Nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a key step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules ( nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase. Classification and structure Although the equilibrium formation of ammonia from molecular hydrogen and nitrogen has an overall negative enthalpy of reaction ( \Delta H^ = -45.2 \ \mathrm \, \mathrm \; \mathrm ), the activation energy is very high ( E_\mathrm = 230-420 \ \mathrm \, \mathrm ). Nitrogenase acts as a catalyst, reducing this energy barr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdate
In chemistry a molybdate is a compound containing an oxoanion with molybdenum in its highest oxidation state of 6. Molybdenum can form a very large range of such oxoanions which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxoanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxoanions range in size from the simplest , found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, , , and ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten. Examples of molybdate anions Examples of molybdate oxoan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdopterin
Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopterin itself contains no molybdenum; rather, this is the name of the ligand (a '' pterin'') that will bind the active metal. After molybdopterin is eventually complexed with molybdenum, the complete ligand is usually called molybdenum cofactor. Molybdopterin consists of a pyranopterin, a complex heterocycle featuring a pyran fused to a pterin ring. In addition, the pyran ring features two thiolates, which serve as ligands in molybdo- and tungstoenzymes. In some cases, the alkyl phosphate group is replaced by an alkyl diphosphate nucleotide. Enzymes that contain the molybdopterin cofactor include xanthine oxidase, DMSO reductase, sulfite oxidase, and nitrate reductase. The only molybdenum-containing enzymes that do not feature molybd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter Jacob Hjelm
Peter (Petter) Jacob Hjelm (2 October 1746 – 7 October 1813) was a Swedish chemist and the first person to isolate the element molybdenum in 1781, four years after its discovery by Swedish chemist Carl Wilhelm Scheele. Working with Molybdic acid, Hjelm chemically reduced molybdenum oxide with carbon in an oxygen-free atmosphere, resulting in carbon dioxide and a near-pure dark metal powder to which he gave the name 'molybdenum'. His first publication on molybdenum appeared in 1790. Childhood Hjelm was born at Sunnerbo in Småland, Sweden in 1746. The son of parish priest Erik Hjelm and Cecilia Cecilia Gistrénia, he was raised in the parish of Göteryd in Älmhult. Career After studying at the University of Uppsala, he received his Ph.D. He became professor at the Mining academy and in 1782 he became Proberare of the Royal Mint, with the job of analyzing minerals to determine their content. From 1784 on he was a member of the Royal Swedish Academy of Sciences T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Element
In the context of nutrition, a mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life. However, the four major structural elements in the human body by weight (oxygen, hydrogen, carbon, and nitrogen), are usually not included in lists of major nutrient minerals (nitrogen is considered a "mineral" for plants, as it often is included in fertilizers). These four elements compose about 96% of the weight of the human body, and major minerals (macrominerals) and minor minerals (also called trace elements) compose the remainder. Nutrient minerals, being elements, cannot be synthesized biochemically by living organisms. Plants get minerals from soil. Most of the minerals in a human diet come from eating plants and animals or from drinking water. As a group, ''minerals'' are one of the four groups of essential nutrients, the others of which are vitamins, essential fatty acids, and essential amino acids. The five major minerals i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the '' metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polythiazyl, polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FeMoco
FeMoco ( cofactor) is the primary cofactor of nitrogenase. Nitrogenase is the enzyme that catalyzes the conversion of atmospheric nitrogen molecules N2 into ammonia (NH3) through the process known as nitrogen fixation. Containing iron and molybdenum, the cofactor is called FeMoco. Its stoichiometry is Fe7MoS9C. Structure The FeMo cofactor is a cluster with composition Fe7MoS9C. Fe is the chemical symbol for the element iron (ferrum), and Mo is the symbol for molybdenum. This large cluster can be viewed as two subunits composed of one Fe4S3 ( iron(III) sulfide) cluster and one MoFe3S3 cluster. The two clusters are linked by three sulfide ligands. The unique iron (Fe) is anchored to the protein by a cysteine. It is also bound to three sulfides, resulting in tetrahedral molecular geometry. The additional six Fe centers in the cluster are each bonded to three sulfides. These six internal Fe centers define a trigonal prismatic arrangement around a central carbide center. The mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmospheric nitrogen is molecular dinitrogen, a relatively nonreactive molecule that is metabolically useless to all but a few microorganisms. Biological nitrogen fixation or ''diazotrophy'' is an important microbials mediated process that converts dinitrogen (N2) gas to ammonia (NH3) using the nitrogenase protein complex (Nif). Nitrogen fixation is essential to life because fixed inorganic nitrogen compounds are required for the biosynthesis of all nitrogen-containing organic compounds, such as amino acids and proteins, nucleoside triphosphates and nucleic acids. As part of the nitrogen cycle, it is essential for agriculture and the manufacture of fertilizer. It is also, indirectly, relevant to the manufacture of all nitrogen chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symbol (chemistry)
Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek vocabulary. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a new Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter temporary symbol may be assigned to a newly synthesized (or not yet synthesized) element. For example, "Uno" was the temporary symbol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead is a chemical element with the Symbol (chemistry), symbol Pb (from the Latin ) and atomic number 82. It is a heavy metals, heavy metal that is density, denser than most common materials. Lead is Mohs scale of mineral hardness#Intermediate hardness, soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable nuclide, stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and base (chemistry), bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist. Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydrogen, and chlorine, among others. Scheele discovered organic acids tartaric, oxalic, uric, lactic, and citric, as well as hydrofluoric, hydrocyanic, and arsenic acids. He preferred speaking German to Swedish his whole life, as German was commonly spoken among Swedish pharmacists.Fors, Hjalmar 2008. Stepping through Science’s Door: C. W. Scheele, from Pharmacist's Apprentice to Man of Science. Ambix 55: 29–49 Biography Scheele was born in Stralsund, in western Pomerania, which at the time was a Swedish Dominion inside the Holy Roman Empire. Scheele's father, Joachim (or Johann) Christian Scheele, was a grain dealer and brewer from a respected Pomeranian family. His mother was Margaretha Eleanore Warnekros. Friends of Scheele's p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Native Metal
A native metal is any metal that is found pure in its metallic form in nature. Metals that can be found as native deposits singly or in alloys include aluminium, antimony, arsenic, bismuth, cadmium, chromium, cobalt, indium, iron, manganese, molybdenum, nickel, niobium, rhenium, selenium, tantalum, tellurium, tin, titanium, tungsten, vanadium, and zinc, as well as the gold group (gold, copper, lead, aluminium, mercury, silver) and the platinum group (platinum, iridium, osmium, palladium, rhodium, ruthenium). Among the alloys found in native state have been brass, bronze, pewter, German silver, osmiridium, electrum, white gold, silver-mercury amalgam, and gold-mercury amalgam. Only gold, silver, copper and the platinum group occur native in large amounts. Over geological time scales, very few metals can resist natural weathering processes like oxidation, so mainly the less reactive metals such as gold and platinum are found as native metals. The others usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |