|
Fusible Alloy
A fusible alloy is a metal alloy capable of being easily fused, i.e. easily meltable, at relatively low temperatures. Fusible alloys are commonly, but not necessarily, eutectic alloys. Sometimes the term "fusible alloy" is used to describe alloys with a melting point below . Fusible alloys in this sense are used for solder. Introduction From a practical view, low-melting alloys can be divided into the following categories: * Mercury-containing alloys *Only alkali metal-containing alloys *Gallium-containing alloys (but neither alkali metal nor mercury) *Only bismuth, lead, tin, cadmium, zinc, indium, and sometimes thallium-containing alloys *Other alloys (rarely used) Some reasonably well-known fusible alloys are Wood's metal, Field's metal, Rose metal, Galinstan, and NaK. Applications Melted fusible alloys can be used as coolants as they are stable under heating and can give much higher thermal conductivity than most other coolants; particularly with alloys made with a high th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the ''metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials regarded as metals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rose Metal
Rose's metal, Rose metal or Rose's alloy is a fusible alloy with a low melting point. Rose's metal consists of 50% bismuth, 25–28% lead and 22–25% tin. Its melting point is between . The alloy does not appreciably contract or expand on solidification, this characteristic being a function of its bismuth percentage. Uses Rose's metal has several common uses: #As a solder. It was used to secure cast iron railings and balusters in pockets in stone bases and steps. #As a heat transfer medium in heating baths. #As a malleable filling to prevent tubes and pipes from crimping when bent. Rose's metal is melted and poured into the tube. It then solidifies in place but remains malleable. This allows the tube or pipe to be bent and reworked without crimping. After the desired shape is achieved the Rose's metal is remelted and removed, leaving the pipe or tube in its modified shape. History It was discovered by the German chemist Valentin Rose the Elder, the grandfather of Heinrich Rose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerrolow 136
Wood's metal, also known as Lipowitz's alloy or by the commercial names Cerrobend, Bendalloy, Pewtalloy and MCP 158, is a metal alloy that is useful for soldering and making custom metal parts, but which is toxic to touch or breathe vapors from. The alloy is named for Barnabas Wood, who first created and patented the alloy in 1860. It is a eutectic, fusible alloy of 50% bismuth, 26.7% lead, 13.3% tin, and 10% cadmium by mass. It has a melting point of approximately . Applications Wood's metal is useful as a low-melting solder, low-temperature casting metal, high-temperature coupling fluid in heat baths, and as a fire-melted valve element in fire sprinkler systems in buildings. Medical gas cylinders A gas cylinder is a pressure vessel for storage and containment of gases at above atmospheric pressure. High-pressure gas cylinders are also called ''bottles''. Inside the cylinder the stored contents may be in a state of compressed gas, vapor ... in the U ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerrolow 117
Wood's metal, also known as Lipowitz's alloy or by the commercial names Cerrobend, Bendalloy, Pewtalloy and MCP 158, is a metal alloy that is useful for soldering and making custom metal parts, but which is toxic to touch or breathe vapors from. The alloy is named for Barnabas Wood, who first created and patented the alloy in 1860. It is a eutectic, fusible alloy of 50% bismuth, 26.7% lead, 13.3% tin, and 10% cadmium by mass. It has a melting point of approximately . Applications Wood's metal is useful as a low-melting solder, low-temperature casting metal, high-temperature coupling fluid in heat baths, and as a fire-melted valve element in fire sprinkler systems in buildings. Medical gas cylinders in the United Kingdom have a Wood's metal seal, which melts in fire, allowing the gas to escape and reducing the risk of gas explosion. Wood's metal is commonly used as a filler when bending thin-walled metal tubes. For this use the tubing is filled with molten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher than water. On Earth, natural rubidium comprises two isotopes: 72% is a stable isotope 85Rb, and 28% is slightly radioactive 87Rb, with a half-life of 48.8 billion years—more than three times as long as the estimated age of the universe. German chemists Robert Bunsen and Gustav Kirchhoff discovered rubidium in 1861 by the newly developed technique, flame spectroscopy. The name comes from the Latin word , meaning deep red, the color of its emission spectrum. Rubidium's compounds have various chemical and electronic applications. Rubidium metal is easily vaporized and has a convenient spectral absorption range, making it a frequent target for laser manipulation of atoms. Rubidium is not a known nutrient for any living organisms. However, r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The first small-scale applications for caesium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solder
Solder (; NA: ) is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces after cooling. Metals or alloys suitable for use as solder should have a lower melting point than the pieces to be joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics. Soft solder typically has a melting point range of , and is commonly used in electronics, plumbing, and sheet metal work. Alloys that melt between are the most commonly used. Soldering performed using alloys with a melting point above is called "hard soldering", "silver soldering", or brazing. In specific proportions, some alloys are eutectic — that is, the alloy's melting point is the lowest possible for a mixture of those components, and co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire Sprinkler
A fire sprinkler or sprinkler head is the component of a fire sprinkler system that discharges water when the effects of a fire have been detected, such as when a predetermined temperature has been exceeded. Fire sprinklers are extensively used worldwide, with over 40 million sprinkler heads fitted each year. In buildings protected by properly designed and maintained fire sprinklers, over 99% of fires were controlled by fire sprinklers alone. History In 1812, British inventor Sir William Congreve patented a manual sprinkler system using perforated pipes along the ceiling. When someone noticed a fire, a valve outside the building could be opened to send water through the pipes. It was not until a short time later that, as a result of a large furniture factory that repeatedly burned down, Hiram Stevens Maxim was consulted on how to prevent a recurrence and invented the first automatic fire sprinkler. It would douse the areas that were on fire and report the fire to the fire st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Boiler
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Steam that is saturated or superheated is invisible; however, "steam" often refers to wet steam, the visible mist or aerosol of water droplets formed as water vapor condenses. Water increases in volume by 1,700 times at standard temperature and pressure; this change in volume can be converted into mechanical work by steam engines such as reciprocating piston type engines and steam turbines, which are a sub-group of steam engines. Piston type steam engines played a central role in the Industrial Revolution and modern steam turbines are used to generate more than 80% of the world's electricity. If liquid water comes in contact with a very hot surface or depressurizes quickly below its vapor pressure, it can create a steam explosion. Type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid (water or gas), which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for medical and industrial use, or for production of weapons-grade plutonium. , the International Atomic Energy Agency reports there are 422 nuclear power reactors and 223 nuclear research reactors in operation around the world. In the early era of nuclear reactors (1940s), a reactor was known as a nuclear pile or atomic pile (so-called because the graphite moderator blocks of the first reactor were placed into a tall pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
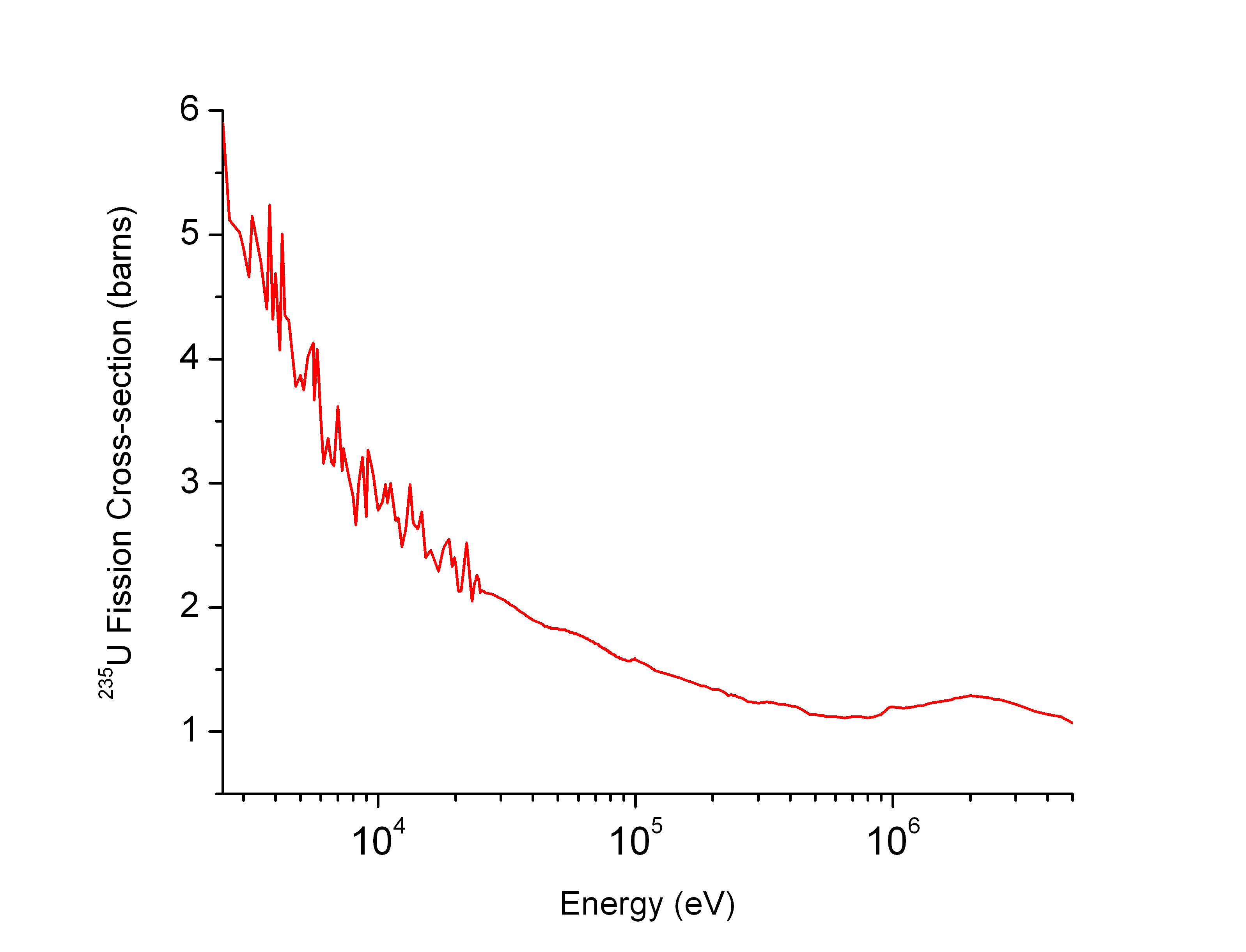
Neutron Cross-section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |