|
Freon 113
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It remains in the atmosphere about 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (where sunlight in the 190-225 nm (UV) range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy more . The Cl• will destroy an average of 100,000 molecules during its atmospheric lifetime of 1–2 years. In some parts of the world, these reactions have significantly thinned the Earth's natural stratospheric ozone layer that shields the biosphere against solar UV radiation; increased UV l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemical formula CCl4. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It is practically incombustible at lower temperatures. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal. Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedron, tetrahedral configuration joined to a central carbon atom by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane. They are also commonly known by the DuPont brand name Freon. The most common representative is dichlorodifluoromethane (R-12 or Freon-12). Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), and solvents. Because CFCs contribute to ozone depletion in the upper atmosphere, the manufacture of such compounds has been phased out under the Montreal Protocol, and they are being replaced with other products such as hydrofluorocarbons (HFCs) including R-410A and R-134a. Structure, properties and production As in simpler alkanes, carbon in the CFCs bond with tetrahedral symmetry. Because the fluorine and chlorine atoms differ greatly in size and effective charge from hydrogen and from each other, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
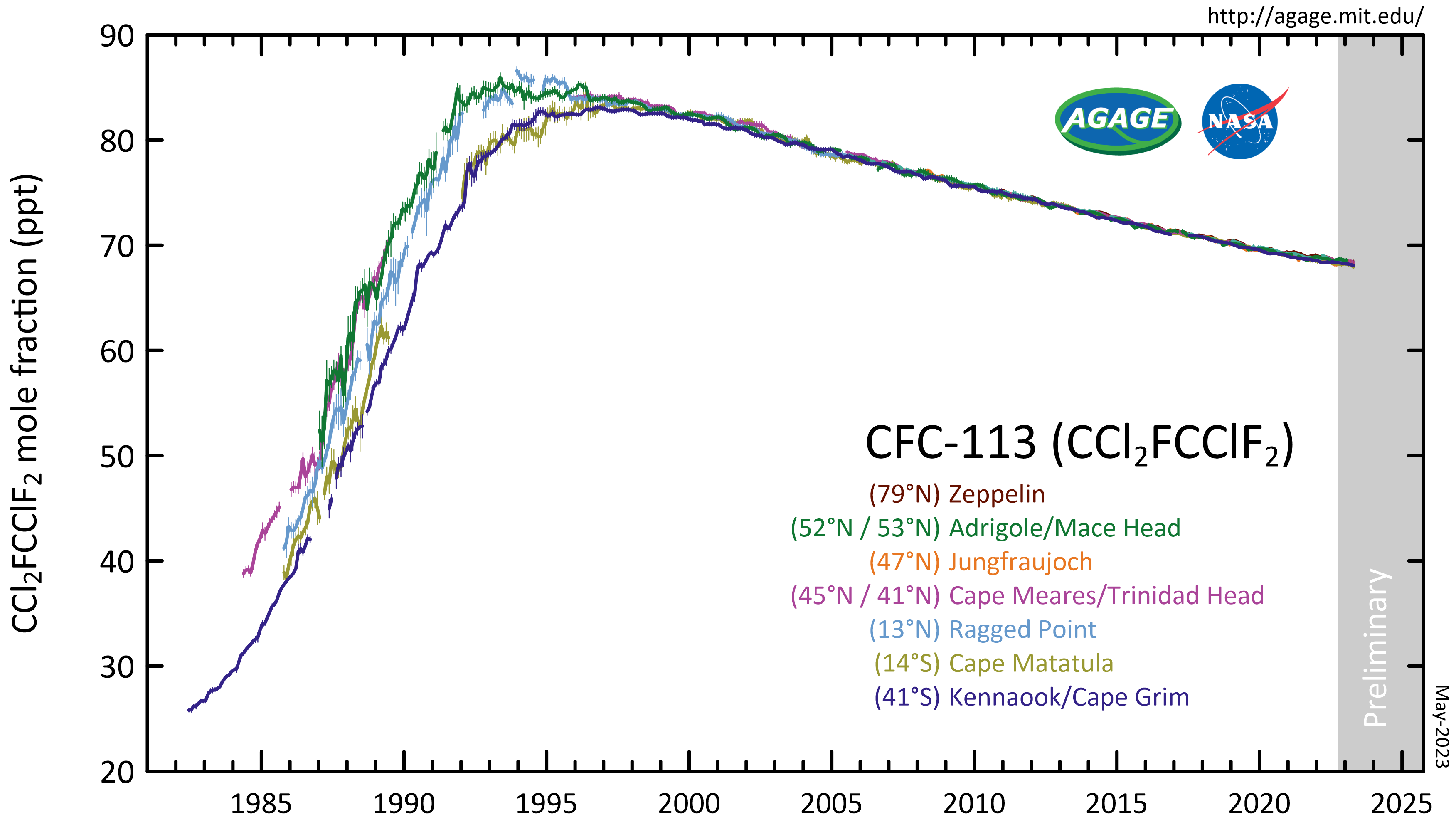
CFC-113 Mm
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It remains in the atmosphere about 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (where sunlight in the 190-225 nm (UV) range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy more . The Cl• will destroy an average of 100,000 molecules during its atmospheric lifetime of 1–2 years. In some parts of the world, these reactions have significantly thinned the Earth's natural stratospheric ozone layer that shields the biosphere against solar UV radiation; increased UV ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hats F113 Global
A hat is a head covering which is worn for various reasons, including protection against weather conditions, ceremonial reasons such as university graduation, religious reasons, safety, or as a fashion accessory. Hats which incorporate mechanical features, such as visors, spikes, flaps, braces or beer holders shade into the broader category of headgear. In the past, hats were an indicator of social status. In the military, hats may denote nationality, branch of service, rank or regiment. Police typically wear distinctive hats such as peaked caps or brimmed hats, such as those worn by the Royal Canadian Mounted Police. Some hats have a protective function. As examples, the hard hat protects construction workers' heads from injury by falling objects, a British police Custodian helmet protects the officer's head, a sun hat shades the face and shoulders from the sun, a cowboy hat protects against sun and rain and an ushanka fur hat with fold-down earflaps keeps the head and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atmosphere Of Earth
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing for liquid water to exist on the Earth's surface, absorbing ultraviolet solar radiation, warming the surface through heat retention (greenhouse effect), and reducing temperature extremes between day and night (the diurnal temperature variation). By mole fraction (i.e., by number of molecules), dry air contains 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other gases. Air also contains a variable amount of water vapor, on average around 1% at sea level, and 0.4% over the entire atmosphere. Air composition, temperature, and atmospheric pressure vary with altitude. Within the atmosphere, air suitable for use in photosynthesis by terrestrial plants and breathing of terrestrial animals is found only in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Michigan
, mottoeng = "Arts, Knowledge, Truth" , former_names = Catholepistemiad, or University of Michigania (1817–1821) , budget = $10.3 billion (2021) , endowment = $17 billion (2021)As of October 25, 2021. , president = Santa Ono , provost = Laurie McCauley , established = , type = Public research university , academic_affiliations = , students = 48,090 (2021) , undergrad = 31,329 (2021) , postgrad = 16,578 (2021) , administrative_staff = 18,986 (2014) , faculty = 6,771 (2014) , city = Ann Arbor , state = Michigan , country = United States , coor = , campus = Midsize City, Total: , including arboretum , colors = Maize & Blue , nickname = Wolverines , sporti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From the planetary surface of the Earth, the average height of the troposphere is in the tropics; in the middle latitudes; and in the high latitudes of the polar regions in winter; thus the average height of the troposphere is . The term ''troposphere'' derives from the Greek words ''tropos'' (rotating) and '' sphaira'' (sphere) indicating that rotational turbulence mixes the layers of air and so determines the structure and the phenomena of the troposphere. The rotational friction of the troposphere against the planetary surface affects the flow of the air, and so forms the planetary boundary layer (PBL) that varies in height from hundreds of meters up to . The measures of the PBL vary according to the latitude, the landform, and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stratosphere
The stratosphere () is the second layer of the atmosphere of the Earth, located above the troposphere and below the mesosphere. The stratosphere is an atmospheric layer composed of stratified temperature layers, with the warm layers of air high in the sky and the cool layers of air in the low sky, close to the planetary surface of the Earth. The increase of temperature with altitude is a result of the absorption of the Sun's ultraviolet (UV) radiation by the ozone layer. The temperature inversion is in contrast to the troposphere, near the Earth's surface, where temperature decreases with altitude. Between the troposphere and stratosphere is the tropopause border that demarcates the beginning of the temperature inversion. Near the equator, the lower edge of the stratosphere is as high as , at midlatitudes around , and at the poles about . Temperatures range from an average of near the tropopause to an average of near the mesosphere. Stratospheric temperatures also vary w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet Radiation
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionize atoms, it can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surfaces with which it comes into contact. For hum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Columbia Encyclopedia
The ''Columbia Encyclopedia'' is a one-volume encyclopedia produced by Columbia University Press and, in the last edition, sold by the Gale Group. First published in 1935, and continuing its relationship with Columbia University, the encyclopedia underwent major revisions in 1950 and 1963; the current edition is the sixth, printed in 2000. It contains over 51,000 articles totaling some 6.5 million words and has also been published in two volumes. An electronic version of the encyclopedia is available, and the ''Columbia Encyclopedia'' is licensed by several different companies for use over the Internet. See also *''Lincoln Library of Essential Information'' * Lists of encyclopedias For lists of encyclopedias, see: * List of encyclopedias by branch of knowledge * List of encyclopedias by date * List of encyclopedias by language * List of online encyclopedias See also * Bibliography of encyclopedias * List of almanacs * ... * List of online encyclopedias References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lexington, Massachusetts
Lexington is a suburban town in Middlesex County, Massachusetts, United States. It is 10 miles (16 km) from Downtown Boston. The population was 34,454 as of the 2020 census. The area was originally inhabited by Native Americans, and was first settled by Europeans in 1641 as a farming community. Lexington is well known as the site of the first shots of the American Revolutionary War, in the Battle of Lexington on April 19, 1775, where the " Shot heard 'round the world" took place. It is home to Minute Man National Historical Park. History Indigenous history Native Americans inhabited the area that would become Lexington for thousands of years prior to European colonization of the Americas, as attested by a woodland era archaeological site near Loring Hill south of the town center. At the time of European contact, the area may have been a border region between Naumkeag or Pawtucket to the northeast, Massachusett to the south, and Nipmuc to the west, though the land was ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrifluoroethylene
Chlorotrifluoroethylene (CTFE) is a chlorofluorocarbon with chemical formula CFCl=CF2. It is commonly used as a refrigerant in cryogenic applications. CTFE has a carbon-carbon double bond and so can be polymerized to form polychlorotrifluoroethylene or copolymerized to produce the plastic ECTFE. PCTFE has the trade name Neoflon PCTFE from Daikin Industries in Japan, and it used to be produced under the trade name Kel-F from 3M Corporation in Minnesota. Production and reactions Chlorotrifluoroethylene is produced commercially by the dechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane with zinc: :CFCl2-CF2Cl + Zn → CClF=CF2 + ZnCl2 In 2012, an estimated 1–10 million pounds were produced commercially in the United States. The thermal dimerization of chlorotrifluoroethylene gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane. Dichlorination of the latter gives hexafluorocyclobutene Hexafluorocyclobutene is the organofluorine compound with the formula (CF2)2(CF)2. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |