|
Forskolin
Forskolin (coleonol) is a labdane diterpene produced by the plant ''Coleus barbatus'' (Blue Spur Flower). Other names include pashanabhedi, Indian coleus, makandi, HL-362, mao hou qiao rui hua. As with other members of the large diterpene class of plant metabolites, forskolin is derived from geranylgeranyl pyrophosphate (GGPP). Forskolin contains some unique functional elements, including the presence of a tetrahydropyran-derived heterocyclic ring. Forskolin is commonly used in laboratory research to increase levels of cyclic AMP by stimulation of adenylate cyclase. Mechanism of action Forskolin is commonly used in biochemistry to raise levels of cyclic AMP (cAMP) in the study and research of cell physiology. Forskolin activates the enzyme adenylyl cyclase and increases intracellular levels of cAMP. cAMP is an important second messenger necessary for the proper biological response of cells to hormones and other extracellular signals. It is required for cell communication in the hyp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic AMP
Cyclic adenosine monophosphate (cAMP, cyclic AMP, or 3',5'-cyclic adenosine monophosphate) is a second messenger important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway. History Earl Sutherland of Vanderbilt University won a Nobel Prize in Physiology or Medicine in 1971 "for his discoveries concerning the mechanisms of the action of hormones", especially epinephrine, via second messengers (such as cyclic adenosine monophosphate, cyclic AMP). Synthesis Cyclic AMP is synthesized from ATP by adenylate cyclase located on the inner side of the plasma membrane and anchored at various locations in the interior of the cell. Adenylate cyclase is ''activated'' by a range of signaling molecules through the activation of adenylate cyclase stimulatory G ( Gs)-protein-coupled receptors. Adenylate cyclase is ''inhibited'' by agonists of adenylat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Adenosine Monophosphate
Cyclic adenosine monophosphate (cAMP, cyclic AMP, or 3',5'-cyclic adenosine monophosphate) is a second messenger important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway. History Earl Sutherland of Vanderbilt University won a Nobel Prize in Physiology or Medicine in 1971 "for his discoveries concerning the mechanisms of the action of hormones", especially epinephrine, via second messengers (such as cyclic adenosine monophosphate, cyclic AMP). Synthesis Cyclic adenosine monophosphate, AMP is synthesized from Adenosine triphosphate, ATP by adenylate cyclase located on the inner side of the plasma membrane and anchored at various locations in the interior of the cell. Adenylate cyclase is ''activated'' by a range of signaling molecules through the activation of adenylate cyclase stimulatory G (Gs alpha subunit, Gs)-protein-coupled recep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coleus Barbatus
''Coleus barbatus'', also known by the synonyms ''Plectranthus barbatus'' and incorrectly ''Coleus forskalaei'' (and other spellings of this epithet), is a tropical perennial plant related to the typical coleus species. It produces forskolin, an extract useful for pharmaceutical preparations and research in cell biology. Name The Brazilian name is (), or , as opposed to the Chilean true boldo; (); (); or (; 'Oxalá's carpet', because of its velvety texture). Taxonomy ''Coleus barbatus'' was first described by Henry Cranke Andrews in 1810 as ''Plectranthus barbatus''. It was transferred to ''Coleus'' by Bentham in 1830. Although ''Coleus'' was previously sunk into ''Plectranthus'', the original binomial was revived in a major study of the subtribe Plectranthinae in 2019. There has been some confusion over the synonyms of this species. ''Plectranthus forskaolaei'' was first described by Vahl in 1790. Vahl's name is illegitimate, because he treats it as a synonym of the earlie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colforsin Daropate
Colforsin daropate is a carboxylic ester derived from the condensation of forskolin (colforsin) with ''N'',''N''-dimethyl-β-alanine. Its water-soluble hydrochloride salt ( NKH 477) is an adenylyl cyclase activator which has been studied for its cardiac selectivity. Its parent compound forskolin (colforsin) is also used to raise levels of cAMP Camp may refer to: Outdoor accommodation and recreation * Campsite or campground, a recreational outdoor sleeping and eating site * a temporary settlement for nomads * Camp, a term used in New England, Northern Ontario and New Brunswick to descri ... in the study of cell physiology. References Acetate esters Vinyl compounds {{pharma-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Labdane
Labdane is a natural bicyclic diterpene. It forms the structural core for a wide variety of natural products collectively known as ''labdanes'' or ''labdane diterpenes''. The labdanes were so named because the first members of the class were originally obtained from labdanum, a resin derived from the gum rockrose. A variety of biological activities have been determined for labdane diterpenes including antibacterial, antifungal, antiprotozoal, and anti-inflammatory activities. Example labdane derivatives * Forskolin * Galanolactone * Isocupressic acid - is an abortifacient component of ''Cupressus macrocarpa''. * Medigenin *Sclareol * Stemodene See also * Abietane Abietane is a diterpene that forms the structural basis for a variety of natural chemical compounds such as abietic acid, carnosic acid, and ferruginol which are collectively known as abietanes or abietane diterpenes. Abietanes are found in the ... References {{reflist Diterpenes Decalins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
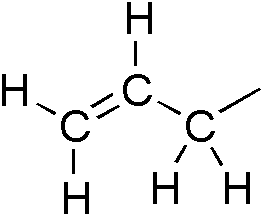
Allyl Group
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphate
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P–O–P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxy Group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Biology International
''Cell Biology International'' is a peer-reviewed scientific journal published by Portland Press for the International Federation for Cell Biology. The journal was established in 1977 as ''Cell Biology International Reports'' () and published by Elsevier, obtaining its current name in 1993. The journal was transferred to Portland Press in 2010. It covers all aspects of cell biology. Abstracting and indexing The journal is abstracted and indexed in: * BIOBASE * BIOSIS * Chemical Abstracts Service * Current Contents * EMBASE * MEDLINE/Index Medicus * Science Citation Index According to the ''Journal Citation Reports'', the journal has a 2010 impact factor of 1.747. See also *Autophagy (journal) *Cell and Tissue Research ''Cell and Tissue Research'' presents regular articles and reviews in the areas of molecular, cell, stem cell biology and tissue engineering. In particular, the journal provides a forum for publishing data that analyze the supracellular, integrati ... Reference ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.edu/hjakubowski/classes/ch331/protstructure/PS_2A3_AA_Charges.html, accessed 12/2/2020 The species formed is the conjugate base of that acid. The complementary process, when a proton is added (transferred) to a Brønsted–Lowry base, is protonation (or hydronation). The species formed is the conjugate acid of that base. A species that can either accept or donate a proton is referred to as amphiprotic. An example is the H2O (water) molecule, which can gain a proton to form the hydronium ion, H3O+, or lose a proton, leaving the hydroxide ion, OH−. The relative ability of a molecule to give up a proton is measured by its p''K''a value. A low p''K''a value indicates that the compound is acidic and will easily give up its proton to a base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. Howe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |