|
Formyl Fluoride
Formyl fluoride is the organic compound with the formula HC(O)F. Preparation HC(O)F was first reported in 1934. Among the many preparations, a typical one involves the reaction of sodium formate with benzoyl fluoride (generated in situ from KHF2 and benzoyl chloride): :HCOONa + C6H5C(O)F → FC(O)H + C6H5COONa Structure The molecule is planar; C-O and C-F distances are 1.18 and 1.34 A, respectively. Reactions HC(O)F decomposes autocatalytically near room temperature to carbon monoxide and hydrogen fluoride: :HC(O)F → HF + CO Because of the compound's sensitivity, reactions are conducted at low temperatures and samples are often stored over anhydrous alkali metal fluorides, e.g. potassium fluoride which absorbs HF. Benzene (and other arenes) react with formyl fluoride in the presence of boron trifluoride to give benzaldehyde. In a related reaction, formyl chloride is implicated in Gattermann-Koch formylation reaction. The reaction of formyl fluoride/BF3 with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freon
Freon ( ) is a registered trademark of the Chemours Company and generic descriptor for a number of halocarbon products. They are stable, nonflammable, low toxicity gases or liquids which have generally been used as refrigerants and as aerosol propellants. These include the chlorofluorocarbons (CFCs) that cause ozone depletion and HCFCs (such as chlorodifluoromethane). Not all refrigerants of this type are labelled as "Freon" since Freon is a brand name for the refrigerants R-12, R-13B1, R-22, R-410A, R-502, and R-503 manufactured by The Chemours Company. Freon emits a strong chemical smell similar to acetone, a nail polish remover component. History The first CFCs were synthesized by Frédéric Swarts in the 1890s. In the late 1920s, a research team was formed by Charles Franklin Kettering in General Motors to find a replacement for the dangerous refrigerants then in use, such as ammonia. The team was headed by Thomas Midgley, Jr. In 1928, they improved the synthesis of CFCs a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arene
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties are understood. The current definition of aromatic compounds does not have any relation with their smell. Heteroarenes are closely related, since at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom. Hydrocarbons without an aromatic ring are called aliphatic. Benzene ring model Benzene, C6H6, is the least complex aromatic hydrocarbon, and it was the first one named as such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid. In organic chemistry, organic acid anhydrides contain the functional group R(CO)O(CO)R'. Organic acid anhydrides often form when one equivalent of water is removed from two equivalents of an organic acid in a dehydration reaction. In inorganic chemistry, an acid anhydride refers to an acidic oxide, an oxide that reacts with water to form an oxyacid (an inorganic acid that contains oxygen or carbonic acid), or with a base to form a salt. Nomenclature The nomenclature of organic acid anhydrides is derived from the names of the constituent carboxylic acids which underwent dehydration to form the compound. In symmetrical acid anhydrides, where only one constituent carboxylic acid was used to form the compound (such as the dehydration of propanoic acid, 2CH3CH2COOH → CH3CH2C(O)OC(O)CH2CH3 + H2O), only the prefix of the original carboxylic acid is used and the suffix "anhydride" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluoroantimonic Acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric acid in terms of its protonating ability measured by Hammett function. It even protonates some hydrocarbons to afford pentacoordinate carbocations ( carbonium ions). Fluoroantimonic acid is corrosive. For example, it cannot be contained directly in glass carboys, as it attacks glass, but can be stored in containers lined with PTFE (Teflon). Chemical composition Fluoroantimonic acid is formed by combining hydrogen fluoride and antimony pentafluoride: :SbF5 + 2 HF + H2F+ The speciation (i.e., the inventory of components) of "fluoroantimonic acid" is complex. Spectroscopic measurements show that fluoroantimonic acid consists of a mixture of HF-solvated protons, – (such as ). Thus, the formula "" is a convenient but oversimplifie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylation
: In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply '' acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Organic synthesis Acetate esters and acetamides are generally prepared by acetylations. Acetylations are often used in making C-acetyl bonds in Friedel-Crafts reactions. Carbanions and their equivalents are susceptible to acetylations. Acetylation reagents Many acetylations are achieved using these three reagents: * Acetic anhydride. This reagent is common in the laboratory; its use cogenerates acetic acid. *Acetyl chloride. This reagent is also common in the laboratory, but its use cogenerates hydrogen chloride, which can be undesirable. *Ketene. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: :H2C=C=O + CH3COOH -> (CH3CO)2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetic Isotope Effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (''kL'') and the heavy (''kH'') isotopically substituted reactants (isotopologues): :\text=\frac This change in reaction rate is a quantum mechanical effect that primarily results from heavier isotopologues having lower vibrational frequencies compared to their lighter counterparts. In most cases, this implies a greater energetic input needed for heavier isotopologues to reach the transition state (or, in rare cases, the dissociation limit), and consequently, a slower reaction rate. The study of kinetic isotope effects can help the elucidation of the reaction mechanism of certain chemical reactions and is occasionally exploited in drug development to improve unfavorable pharmacokinetics by protecting m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). (Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.) The name ''deuterium'' is derived from the Greek , meaning "second", to denot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
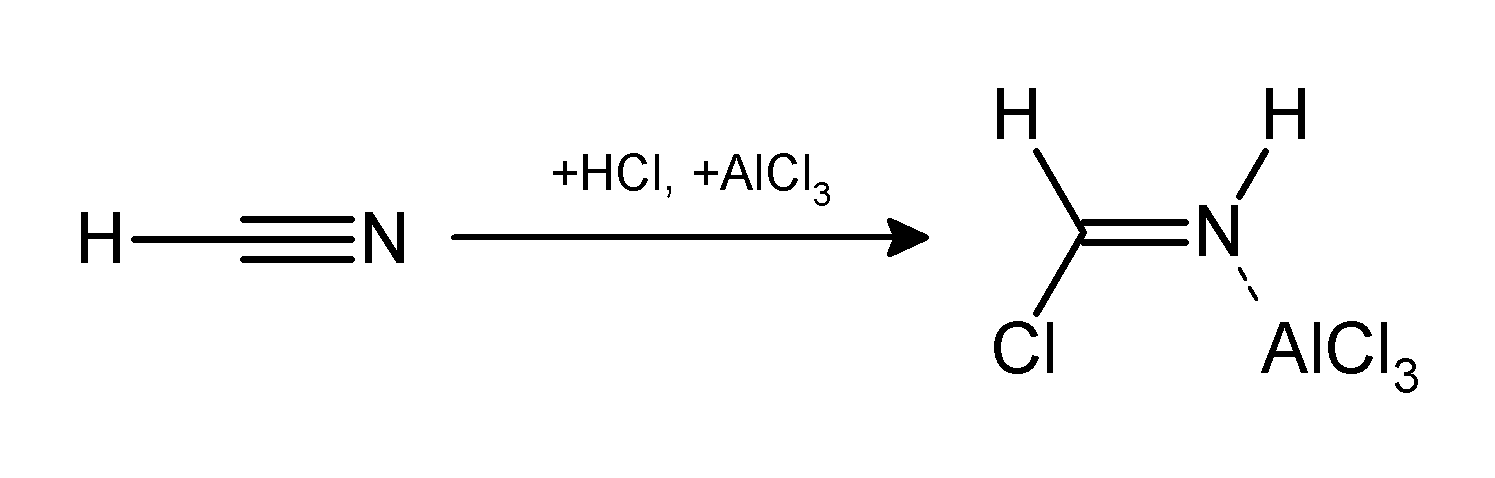
Gattermann-Koch Reaction
The Gattermann reaction, (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as AlCl3. It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction. Modifications have shown that it is possible to use sodium cyanide or cyanogen bromide in place of hydrogen cyanide. The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide. Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN. The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst ''in-situ''. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene. Gattermann–Koch reaction The Gattermann–Koch reaction, nam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



_(2).png)