|
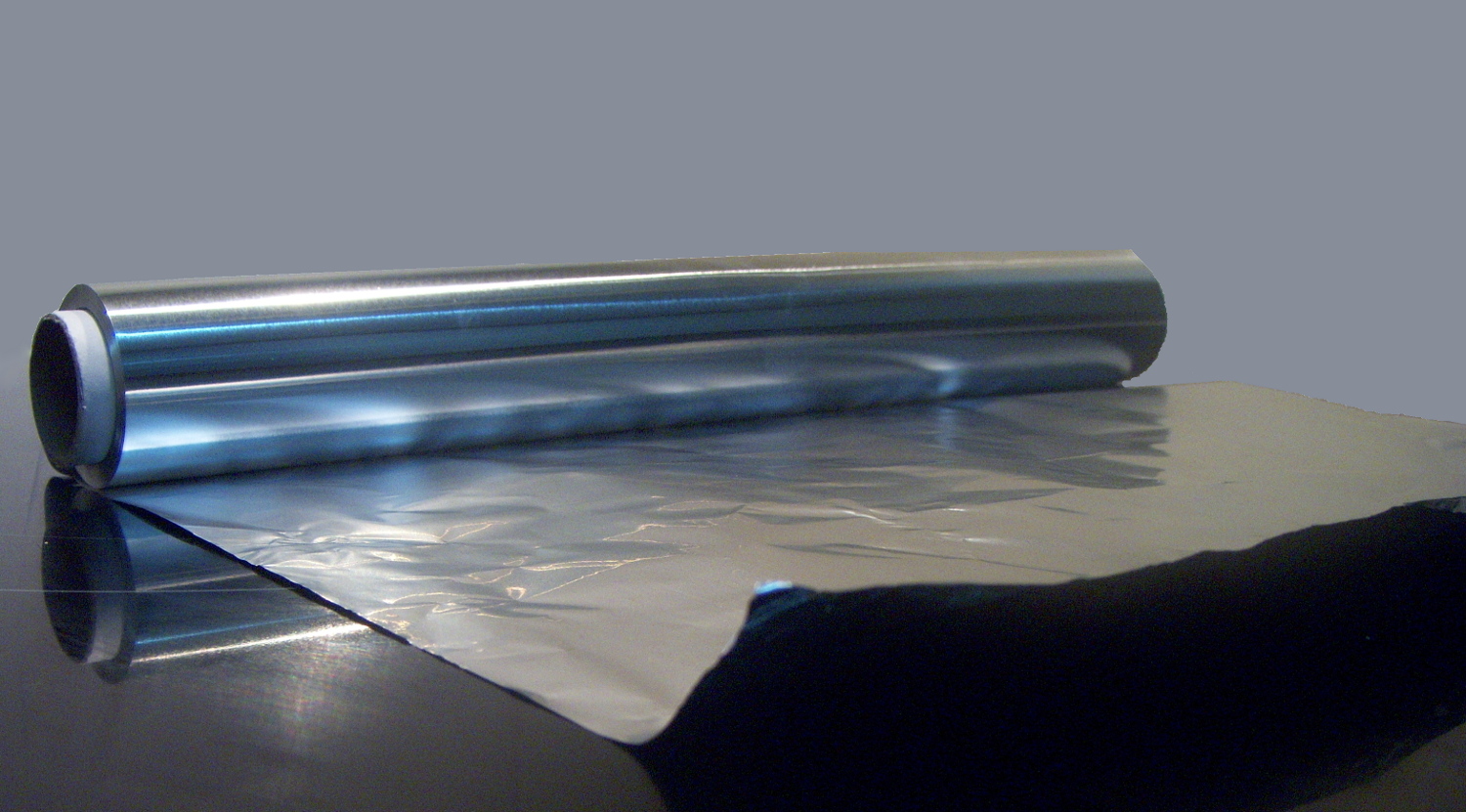
Foil (chemistry)
A foil is a very thin sheet of metal, typically made by hammering or rolling.Britannica, The Editors of Encyclopaedia. "foil". Encyclopedia Britannica, 6 Nov. 2008https://www.britannica.com/technology/foil-metallurgy.Accessed 11 September 2022. Foils are most easily made with malleable metal, such as aluminium, copper, tin, and gold. Foils usually bend under their own weight and can be torn easily. For example, aluminium foil is usually about 1/1000 inch (0.03 mm), whereas gold (more malleable than aluminium) can be made into foil only a few atoms thick, called gold leaf. Extremely thin foil is called metal leaf. Leaf tears very easily and must be picked up with special brushes. Foil is commonly used in household applications. It is also useful in survival situations, in the form of a "space blanket", where the reflective surface reduces the degree of hypothermia caused by thermal radiation. See also * Aluminium foil * Tin foil * Gold leaf * Metal leaf A metal leaf, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the ''metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials regarded as metals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malleable
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stress before failure. Ductility is an important consideration in engineering and manufacturing. It defines a material's suitability for certain manufacturing operations (such as cold working) and its capacity to absorb mechanical overload.. Some metals that are generally described as ductile include gold and copper. However, not all metals experience ductile failure as some can be characterized with brittle failure like cast iron. Polymers generally can be viewed as ductile materials as they typically allow for plastic deformation. Malleability, a similar mechanical property, is characterized by a material's ability to deform plastically without failure under compressive stress. Historically, materials were considered malleable if they were am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile metal in a pure form. Chemically, gold is a transition metal and a group 11 element. It is one of the least reactive chemical elements and is solid under standard conditions. Gold often occurs in free elemental ( native state), as nuggets or grains, in rocks, veins, and alluvial deposits. It occurs in a solid solution series with the native element silver (as electrum), naturally alloyed with other metals like copper and palladium, and mineral inclusions such as within pyrite. Less commonly, it occurs in minerals as gold compounds, often with tellurium (gold tellurides). Gold is resistant to most acids, though it does dissolve in aqua regia (a mixture of nitric acid and hydrochloric acid), forming a soluble tetrachloroaurate anion. Gold is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Foil
Aluminium foil (or aluminum foil in North American English; often informally called tin foil) is aluminium prepared in thin metal leaves with a thickness less than ; thinner gauges down to are also commonly used. Standard household foil is typically thick, and heavy duty household foil is typically . The foil is pliable, and can be readily bent or wrapped around objects. Thin foils are fragile and are sometimes laminated with other materials such as plastics or paper to make them stronger and more useful. Annual production of aluminium foil was approximately in Europe and in the U.S."Foil & Packaging" . The Aluminum Association (USA). in 2003. Approximately 75% of aluminium foil is used for |
Atoms
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold Leaf
Gold leaf is gold that has been hammered into thin sheets (usually around 0.1 µm thick) by goldbeating and is often used for gilding. Gold leaf is available in a wide variety of karats and shades. The most commonly used gold is 22-karat yellow gold. Gold leaf is a type of metal leaf, but the term is rarely used when referring to gold leaf. The term ''metal leaf'' is normally used for thin sheets of metal of any color that do not contain any real gold. Pure gold is 24 karat. Real, yellow gold leaf is approximately 91.7% pure (i.e. 22-karat) gold. Silver-colored white gold is about 50% pure gold. Layering gold leaf over a surface is called gold leafing or gilding. Traditional water gilding is the most difficult and highly regarded form of gold leafing. It has remained virtually unchanged for hundreds of years and is still done by hand. In art Gold leaf is sometimes used in art in a "raw" state, without a gilding process. In cultures including the European Bronze Age it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Leaf
A metal leaf, also called composition leaf or schlagmetal, is a thin foil used for gilding and other forms of decoration. Metal leaves can come in many different shades. Some metal leaves may look like gold leaf but do not contain any real gold. This type of metal leaf is often referred to as imitation leaf. Metal leaves are usually made of gold (including many alloys), silver, copper, aluminium, brass (sometimes called "Dutch metal" typically 85% Copper and 15% zinc) or palladium, sometimes also platinum. Vark is a type of silver leaf used for decoration in Indian cuisine. Goldbeating, the technique of producing metal leaves, has been known for more than 5,000 years. A small gold nugget 5 mm in diameter can be expanded to about 20,000 times its initial surface through hammering, producing a gold foil surface of about one half square meter with a thickness of 0.2–0.3 μm. Nanjing gold leaf forging technique History This is a traditional handicraft in Nanjing, produced a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Blanket
A space blanket (also known as a Mylar blanket, emergency blanket, first aid blanket, safety blanket, thermal blanket, weather blanket, heat sheet, foil blanket, or shock blanket) is an especially low-weight, low-bulk blanket made of heat-reflective, thin, plastic sheeting. They are used on the exterior surfaces of spacecraft for thermal control, as well as by people. Their design reduces the heat loss in a person's body, which would otherwise occur due to thermal radiation, water evaporation, or convection. Their low weight and compact size before unfurling make them ideal when space or weight are at a premium. They may be included in first aid kits and with camping equipment. Lost campers and hikers have an additional possible benefit: the shiny surface flashes in the sun, allowing its use as an improvised distress beacon for searchers and as a method of signalling over long distances to other people. Manufacturing First developed by NASAs Marshall Space Flight Center in 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypothermia
Hypothermia is defined as a body core temperature below in humans. Symptoms depend on the temperature. In mild hypothermia, there is shivering and mental confusion. In moderate hypothermia, shivering stops and confusion increases. In severe hypothermia, there may be hallucinations and paradoxical undressing, in which a person removes their clothing, as well as an increased risk of the heart stopping. Hypothermia has two main types of causes. It classically occurs from exposure to cold weather and cold water immersion. It may also occur from any condition that decreases heat production or increases heat loss. Commonly, this includes alcohol intoxication but may also include low blood sugar, anorexia and advanced age. Body temperature is usually maintained near a constant level of through thermoregulation. Efforts to increase body temperature involve shivering, increased voluntary activity, and putting on warmer clothing. Hypothermia may be diagnosed based on either a person ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |