|
Foam Core Sandwich
Foams are materials formed by trapping pockets of gas in a liquid or solid. A bath sponge and the head on a glass of beer are examples of foams. In most foams, the volume of gas is large, with thin films of liquid or solid separating the regions of gas. Soap foams are also known as suds. Solid foams can be closed-cell or open-cell. In closed-cell foam, the gas forms discrete pockets, each completely surrounded by the solid material. In open-cell foam, gas pockets connect to each other. A bath sponge is an example of an open-cell foam: water easily flows through the entire structure, displacing the air. A sleeping mat is an example of a closed-cell foam: gas pockets are sealed from each other so the mat cannot soak up water. Foams are examples of dispersed media. In general, gas is present, so it divides into gas bubbles of different sizes (i.e., the material is polydisperse)—separated by liquid regions that may form films, thinner and thinner when the liquid phase drain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Foam - Big
Foams are materials formed by trapping pockets of gas in a liquid or solid. A bath sponge and the head on a glass of beer are examples of foams. In most foams, the volume of gas is large, with thin films of liquid or solid separating the regions of gas. Soap foams are also known as suds. Solid foams can be closed-cell or open-cell. In closed-cell foam, the gas forms discrete pockets, each completely surrounded by the solid material. In open-cell foam, gas pockets connect to each other. A bath sponge is an example of an open-cell foam: water easily flows through the entire structure, displacing the air. A sleeping mat is an example of a closed-cell foam: gas pockets are sealed from each other so the mat cannot soak up water. Foams are examples of dispersed media. In general, gas is present, so it divides into gas bubbles of different sizes (i.e., the material is polydisperse)—separated by liquid regions that may form films, thinner and thinner when the liquid phase dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minimal Surface
In mathematics, a minimal surface is a surface that locally minimizes its area. This is equivalent to having zero mean curvature (see definitions below). The term "minimal surface" is used because these surfaces originally arose as surfaces that minimized total surface area subject to some constraint. Physical models of area-minimizing minimal surfaces can be made by dipping a wire frame into a soap solution, forming a soap film, which is a minimal surface whose boundary is the wire frame. However, the term is used for more general surfaces that may self-intersect or do not have constraints. For a given constraint there may also exist several minimal surfaces with different areas (for example, see minimal surface of revolution): the standard definitions only relate to a local optimum, not a global optimum. Definitions Minimal surfaces can be defined in several equivalent ways in R3. The fact that they are equivalent serves to demonstrate how minimal surface theory lies at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (physics)
In physics, work is the energy transferred to or from an object via the application of force along a displacement. In its simplest form, for a constant force aligned with the direction of motion, the work equals the product of the force strength and the distance traveled. A force is said to do ''positive work'' if when applied it has a component in the direction of the displacement of the point of application. A force does ''negative work'' if it has a component opposite to the direction of the displacement at the point of application of the force. For example, when a ball is held above the ground and then dropped, the work done by the gravitational force on the ball as it falls is positive, and is equal to the weight of the ball (a force) multiplied by the distance to the ground (a displacement). If the ball is thrown upwards, the work done by its weight is negative, and is equal to the weight multiplied by the displacement in the upwards direction. When the force is con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane is dependent on the amount of deformation of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pickering Emulsion
A Pickering emulsion is an emulsion that is stabilized by solid particles (for example colloidal silica) which adsorb onto the interface between the water and oil phases. Typically, the emulsions are either water-in-oil or oil-in-water emulsions, but other more complex systems such as water-in-water, oil-in-oil, water-in-oil-in-water, and oil-in-water-in-oil also do exist. Pickering emulsions were named after S.U. Pickering, who described the phenomenon in 1907, although the effect was first recognized by Walter Ramsden in 1903. If oil and water are mixed and small oil droplets are formed and dispersed throughout the water (oil-in-water emulsion), eventually the droplets will coalesce to decrease the amount of energy in the system. However, if solid particles are added to the mixture, they will bind to the surface of the interface and prevent the droplets from coalescing, making the emulsion more stable. Particle properties such as hydrophobicity, shape, and size, as well as t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphiphilic
An amphiphile (from the Greek αμφις amphis, both, and φιλíα philia, love, friendship), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'') properties. Such a compound is called amphiphilic or amphipathic. Common amphiphilic substances are soaps, detergents, and lipoproteins. The phospholipid amphiphiles are the major structural component of cell membranes. Amphiphiles are the basis for a number of areas of research in chemistry and biochemistry, notably that of lipid polymorphism. Organic compounds containing hydrophilic groups at both ends of the molecule are called bolaamphiphilic. The micelles they form in the aggregate are prolate. Structure The lipophilic group is typically a large hydrocarbon moiety, such as a long chain of the form CH3(CH2)n, with n > 4. The hydrophilic group falls into one of the following categories: # charged groups #* anionic. Examples, with the lipophilic part of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lamella (materials)
A ''lamella'' (plural ''lamellae'') is a small plate or flake, from the Latin, and may also be used to refer to collections of fine sheets of material held adjacent to one another, in a gill-shaped structure, often with fluid in between though sometimes simply a set of 'welded' plates. The term is used in biological contexts to describe thin membranes of plates of tissue. In context of materials science, the microscopic structures in bone and nacre are called lamellae. Moreover, the term lamella is often used as a way to describe crystal structure of some materials. Uses of the term In surface chemistry (especially mineralogy and materials science), lamellar structures are fine layers, alternating between different materials. They can be produced by chemical effects (as in eutectic solidification), biological means, or a deliberate process of lamination, such as pattern welding. Lamellae can also describe the layers of atoms in the crystal lattices of materials such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metastable
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy. A ball resting in a hollow on a slope is a simple example of metastability. If the ball is only slightly pushed, it will settle back into its hollow, but a stronger push may start the ball rolling down the slope. Bowling pins show similar metastability by either merely wobbling for a moment or tipping over completely. A common example of metastability in science is isomerisation. Higher energy isomers are long lived because they are prevented from rearranging to their preferred ground state by (possibly large) barriers in the potential energy. During a metastable state of finite lifetime, all state-describing parameters reach and hold stationary values. In isolation: *the state of least energy is the only one the system will inhabit for an indefinite length of time, until more external energy is added to the system (unique "ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plateau's Laws
Plateau's laws describe the structure of soap films. These laws were formulated in the 19th century by the Belgian physicist Joseph Plateau from his experimental observations. Many patterns in nature are based on foams obeying these laws. Laws for soap films Plateau's laws describe the shape and configuration of soap films as follows: # Soap films are made of entire (unbroken) smooth surfaces. # The mean curvature of a portion of a soap film is everywhere constant on any point on the same piece of soap film. # Soap films always meet in threes along an edge called a Plateau border, and they do so at an angle of arccos(−) = 120°. # These Plateau borders meet in fours at a vertex, at the tetrahedral angle of arccos(−) ≈ 109.47°. Configurations other than those of Plateau's laws are unstable, and the film will quickly tend to rearrange itself to conform to these laws. That these laws hold for minimal surfaces was proved mathematically by Jean Taylor using geom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
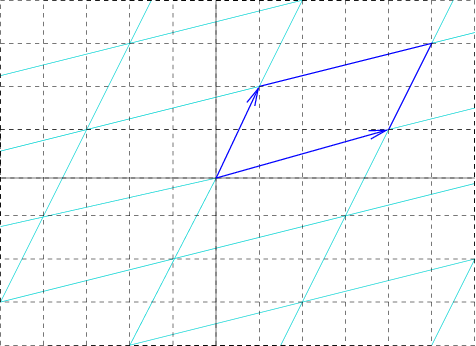
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |






.jpg)