|
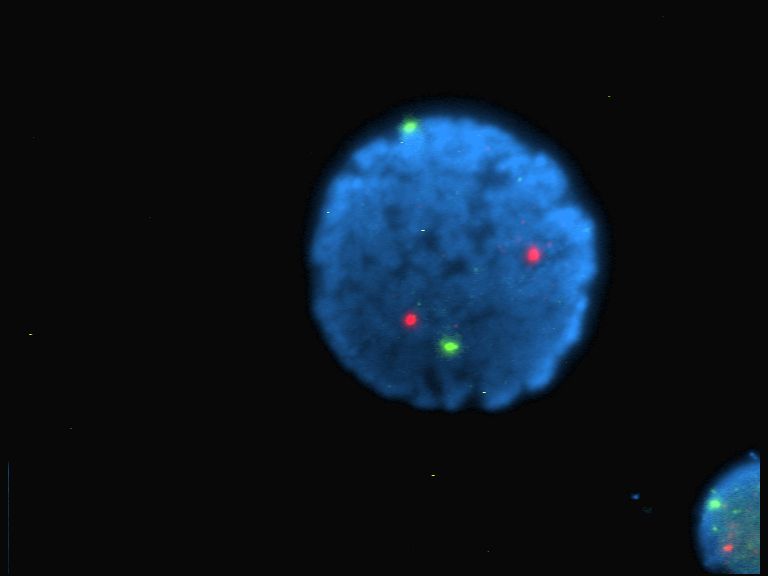
Fluorophor
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to a macromolecule, serving as a marker (or dye, or tag, or reporter) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, i.e., fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores. Fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Förster Resonance Energy Transfer
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules ( chromophores). A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling. The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, making FRET extremely sensitive to small changes in distance. Measurements of FRET efficiency can be used to determine if two fluorophores are within a certain distance of each other. Such measurements are used as a research tool in fields including biology and chemistry. FRET is analogous to near-field communication, in that the radius of interaction is much smaller than the wavelength of light emitted. In the near-field region, the excited chromophore e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence Microscope
A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence microscope" refers to any microscope that uses fluorescence to generate an image, whether it is a simple set up like an epifluorescence microscope or a more complicated design such as a confocal microscope, which uses optical sectioning to get better resolution of the fluorescence image. Principle The specimen is illuminated with light of a specific wavelength (or wavelengths) which is absorbed by the fluorophores, causing them to emit light of longer wavelengths (i.e., of a different color than the absorbed light). The illumination light is separated from the much weaker emitted fluorescence through the use of a spectral emission filter. Typical components of a fluorescence microscope are a light source (xenon arc lamp or mercury-vapor lamp are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
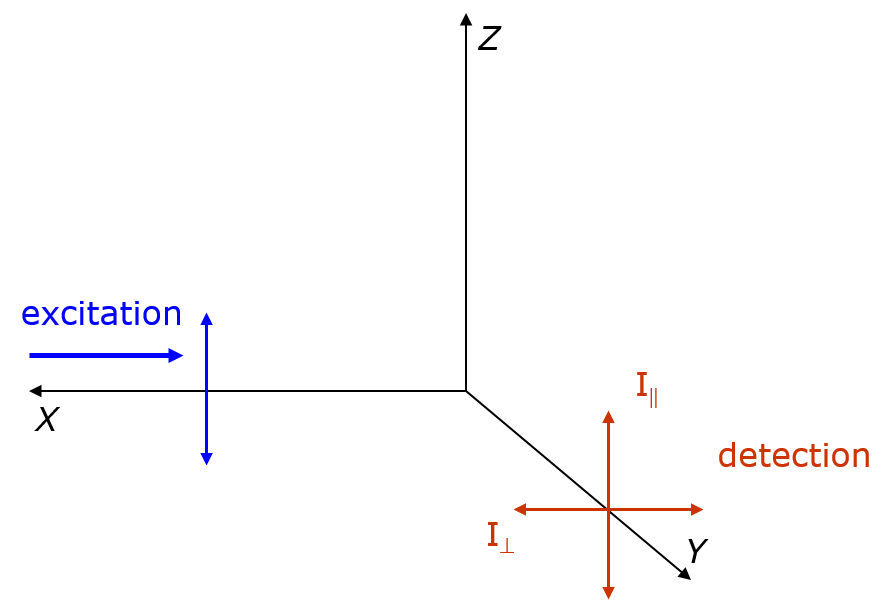
Fluorescence Anisotropy
Fluorescence anisotropy or fluorescence polarization is the phenomenon where the light emitted by a fluorophore has unequal intensities along different axes of polarization. Early pioneers in the field include Aleksander Jablonski, Gregorio Weber, and Andreas Albrecht. The principles of fluorescence polarization and some applications of the method are presented in Lakowicz's book.Lakowicz, J.R., 2006. ''Principles of Fluorescence Spectroscopy'' (3rd ed., Springer. Chapter 10-12 deal with fluorescence polarization spectroscopy.) Definition of fluorescence anisotropy The anisotropy (r) of a light source is defined as the ratio of the polarized component to the total intensity (I_T): r=\frac When the excitation is polarized along the z-axis, emission from the fluorophore is symmetric around the z-axis(Figure). Hence statistically we have I_x=I_y. As I_y=I_, and I_z=I_, we have r=\frac=\frac. Principle – Brownian motion and photoselection In fluorescence, a molecule absorbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence Spectroscopy
Fluorescence spectroscopy (also known as fluorimetry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores. Devices that measure fluorescence are called fluorometers. Theory Molecules have various states referred to as energy levels. Fluorescence spectroscopy is primarily concerned with electronic and vibrational states. Generally, the species being examined has a ground electronic state (a low energy state) of interest, and an excited electronic state of higher energy. Within each of these elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Dots
Quantum dots (QDs) are semiconductor particles a few nanometres in size, having optical and electronic properties that differ from those of larger particles as a result of quantum mechanics. They are a central topic in nanotechnology. When the quantum dots are illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conductance band. The excited electron can drop back into the valence band releasing its energy as light. This light emission ( photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the conductance band and the valence band, or the transition between discrete energy states when band structure is no longer a good definition in QDs. In the language of materials science, nanoscale semiconductor materials tightly confine eith ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quenching (fluorescence)
Quenching refers to any process which decreases the fluorescence intensity of a given substance. A variety of processes can result in quenching, such as excited state reactions, energy transfer, complex-formation and collisional quenching. As a consequence, quenching is often heavily dependent on pressure and temperature. Molecular oxygen, iodide ions and acrylamide are common chemical quenchers. The chloride ion is a well known quencher for quinine fluorescence. Quenching poses a problem for non-instant spectroscopic methods, such as laser-induced fluorescence. Quenching is made use of in optode sensors; for instance the quenching effect of oxygen on certain ruthenium complexes allows the measurement of oxygen saturation in solution. Quenching is the basis for Förster resonance energy transfer (FRET) assays. Quenching and dequenching upon interaction with a specific molecular biological target is the basis for activatable optical contrast agents for molecular imaging. Many dyes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanine
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV. Chemically, cyanines are a conjugated system between two nitrogen atoms; in each resonance structure, exactly one nitrogen atom is oxidized to an iminium. Typically, they form part of a nitrogenous heterocyclic system. The main application for cyanine dyes is in biological labeling. Nevertheless, there is a wide literature on both their synthesis and uses, and cyanines are common in some CD and DVD media. Structure Cyanines have been classified in many ways: * ''Streptocyanines'' or ''open chain cyanines'': : R2N+=CH H=CH'n''-NR2 (I) * ''Hemicyanines'': : Aryl=N+=CH H=CH'n''-NR2 (II) * ''Closed chain cyanines'': :Aryl=N+=CH H=CH'n''-N=Aryl (III) Additionally, these classes are recognized: * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications. The color of its aqueous solutions is green by reflection and orange by transmission (its spectral properties are dependent on pH of the solution), as can be noticed in bubble levels, for example, in which fluorescein is added as a colorant to the alcohol filling the tube in order to increase the visibility of the air bubble contained within (thus enhancing the precision of the instrument). More concentrated solutions of fluorescein can even appear red (because under these conditions nearly all incident emission is re-absorbed by the solution). It is on the World Health Organization's List of Essential Medicines. Uses Fluorescein sodium, the sodium salt of fluorescein, is used extensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodamine
Rhodamine is a family of related dyes, a subset of the triarylmethane dyes. They are derivatives of xanthene. Important members of the rhodamine family are Rhodamine 6G, Rhodamine 123, and Rhodamine B. They are mainly used to dye paper and inks, but they lack the lightfastness for fabric dyeing. Use Aside from their major applications, they are often used as a tracer dye, e.g. to determine the rate and direction of flow and transport of water. Rhodamine dyes fluoresce and can thus be detected easily and inexpensively with instruments called fluorometers. Rhodamine dyes are used extensively in biotechnology applications such as fluorescence microscopy, flow cytometry, fluorescence correlation spectroscopy and ELISA. Rhodamine 123 is used in biochemistry to inhibit mitochondrion function. Rhodamine 123 appears to bind to the mitochondrial membranes and inhibit transport processes, especially the electron transport chain, thus slowing down cellular respiration. It is a substra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescein Isothiocyanate
Fluorescein isothiocyanate (FITC) is a derivative of fluorescein used in wide-ranging applications including flow cytometry. First described in 1942, FITC is the original fluorescein molecule functionalized with an isothiocyanate reactive group (−N=C=S), replacing a hydrogen atom on the bottom ring of the structure. It is typically available as a mixture of isomers, fluorescein 5-isothiocyanate (5-FITC) and fluorescein 6-isothiocyanate (6-FITC). FITC is reactive towards nucleophiles including amine and sulfhydryl groups on proteins. It was synthesized by Robert Seiwald and Joseph Burckhalter in 1958. A succinimidyl-ester functional group attached to the fluorescein core, creating "NHS-fluorescein", forms another common amine reactive derivative that has much greater specificity toward primary amines in the presence of other nucleophiles. FITC has excitation and emission spectrum peak wavelengths of approximately 495 nm and 519 nm, giving it a green color. Like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Efficiency
The term quantum efficiency (QE) may apply to incident photon to converted electron (IPCE) ratio of a photosensitive device, or it may refer to the TMR effect of a Magnetic Tunnel Junction. This article deals with the term as a measurement of a device's electrical sensitivity to light. In a charge-coupled device (CCD) or other photodetector, it is the ratio between the number of charge carriers collected at either terminal and the number of photons hitting the device's photoreactive surface. As a ratio, QE is dimensionless, but it is closely related to the responsivity, which is expressed in amps per watt. Since the energy of a photon is inversely proportional to its wavelength, QE is often measured over a range of different wavelengths to characterize a device's efficiency at each photon energy level. For typical semiconductor photodetectors, QE drops to zero for photons whose energy is below the band gap. A photographic film typically has a QE of much less than 10%, while C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |