|
Flufenamic Acid
Flufenamic acid (FFA) is a member of the anthranilic acid derivatives (or fenamate) class of nonsteroidal anti-inflammatory drugs (NSAIDs). Like other members of the class, it is a cyclooxygenase (COX) inhibitor, preventing the formation of prostaglandins. FFA is known to bind to and reduce the activity of prostaglandin F synthase and activate TRPC6. It is not widely used in humans as it has a high rate (30–60%) of gastrointestinal side effects. It is generally not available in the US.NIH LiverTox DatabasMefenamic AcidLast updated June 23, 2015. Page accessed July 3, 2015. Quote: "(fenamates generally not available in the United States, such as tolfenamic acid and flufenamic acid)" It is available in some Asian and European countries as a generic drug. Scientists led by Claude Winder from Parke-Davis invented FFA in 1963, along with fellow members of the class, mefenamic acid in 1961 and meclofenamic acid Meclofenamic acid (used as meclofenamate sodium, brand name Meclo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TRPC6
Transient receptor potential cation channel, subfamily C, member 6, also known as TRPC6, is a human gene encoding a protein of the same name. TRPC6 is a transient receptor potential channel of the classical TRPC subfamily. It has been associated with depression and anxiety (see below), as well as with focal segmental glomerulosclerosis (FSGS). Interactions TRPC6 has been shown to interact with: * FYN, * TRPC2, and * TRPC3. Ligands Two of the primary active constituents responsible for the antidepressant and anxiolytic benefits of ''Hypericum perforatum'', also known as St. John's Wort, are hyperforin and adhyperforin. These compounds are inhibitors of the reuptake of serotonin, norepinephrine, dopamine, γ-aminobutyric acid, and glutamate, and they are reported to exert these effect Effect may refer to: * A result or change of something ** List of effects ** Cause and effect, an idiom describing causality Pharmacy and pharmacology * Drug effect, a change resulti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
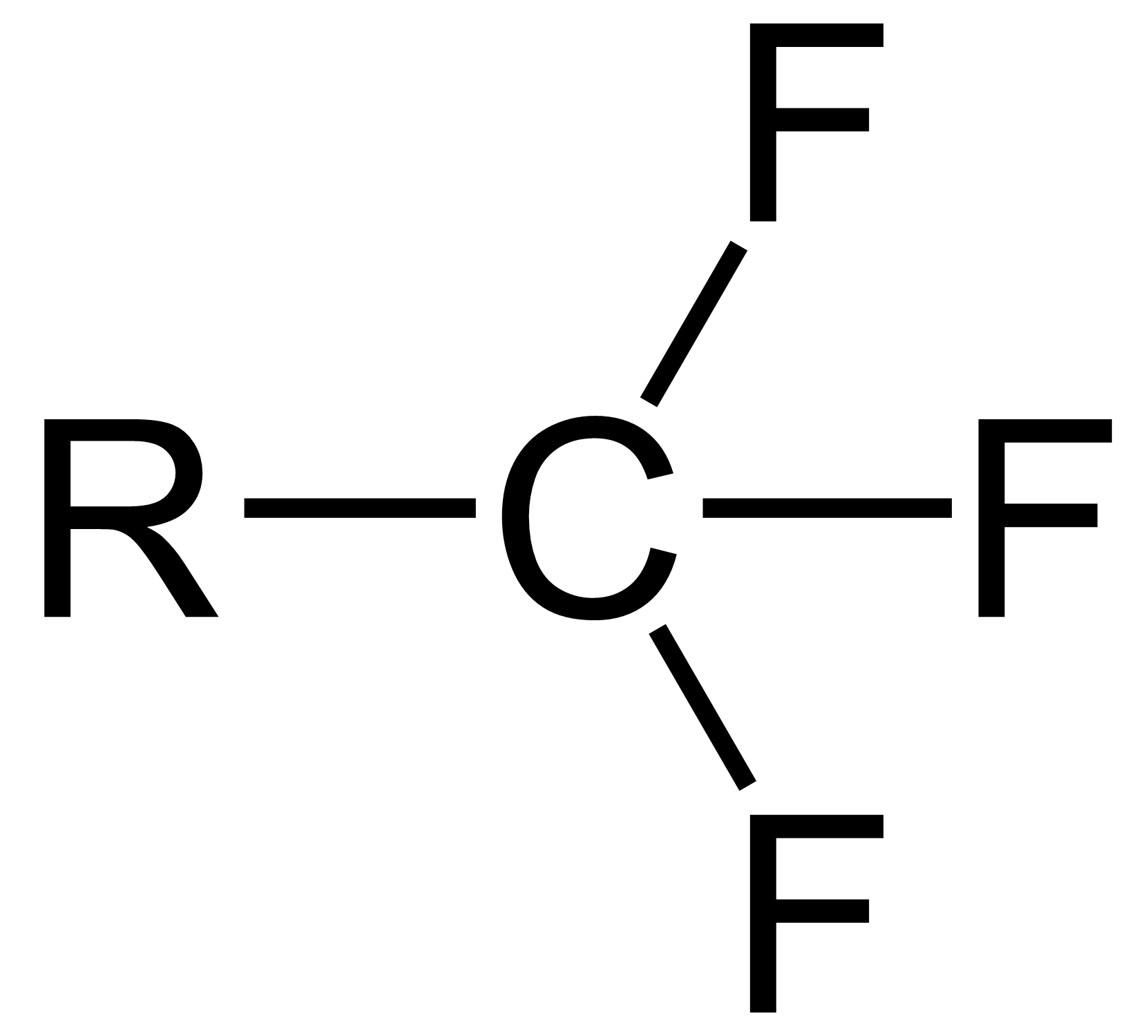
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthranilic Acids )
{{Chemistry index ...
Aminobenzoic acid (a benzoic acid with an amino group) can refer to: * 4-Aminobenzoic acid (''p''-aminobenzoic acid or ''para''-aminobenzoic acid) * 3-Aminobenzoic acid (''m''-aminobenzoic acid or ''meta''-aminobenzoic acid) * 2-aminobenzoic acid (''o''-aminobenzoic acid or ''ortho''-aminobenzoic acid, Anthranilic acid Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ''ortho''-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacodynamics
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs (especially pharmaceutical drugs). The effects can include those manifested within animals (including humans), microorganisms, or combinations of organisms (for example, infection). Pharmacodynamics and pharmacokinetics are the main branches of pharmacology, being itself a topic of biology interested in the study of the interactions between both endogenous and exogenous chemical substances with living organisms. In particular, pharmacodynamics is the study of how a drug affects an organism, whereas pharmacokinetics is the study of how the organism affects the drug. Both together influence dosing, benefit, and adverse effects. Pharmacodynamics is sometimes abbreviated as PD and pharmacokinetics as PK, especially in combined reference (for example, when speaking of PK/PD models). Pharmacodynamics places particular emphasis on dose–response relationships, that is, the relationships between d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meclofenamic Acid
Meclofenamic acid (used as meclofenamate sodium, brand name Meclomen) is a drug used for joint, muscular pain, arthritis and dysmenorrhea. It is a member of the anthranilic acid derivatives (or fenamate) class of nonsteroidal anti-inflammatory drugs (NSAIDs) and was approved by the US FDA in 1980. Like other members of the class, it is a cyclooxygenase (COX) inhibitor, preventing the formation of prostaglandins. Scientists led by Claude Winder from Parke-Davis invented meclofenamate sodium in 1964, along with fellow members of the class, mefenamic acid in 1961 and flufenamic acid in 1963. Patents on the drug expired in 1985 and several generics were introduced in the US, but as of July 2015 only Mylan still sold it. It is not widely used in humans as it has a high rate (30-60%) rate of gastrointestinal side effects. Adverse effects In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mefenamic Acid
Mefenamic acid is a member of the anthranilic acid derivatives (or fenamate) class of nonsteroidal anti-inflammatory drugs (NSAIDs), and is used to treat mild to moderate pain. Its name derives from its systematic name, dimethylphenylaminobenzoic acid. It was discovered and brought to market by Parke-Davis as Ponstel in the 1960s. It became generic in the 1980s and is available worldwide under many brand names such as ''Meftal''. Medical uses Mefenamic acid is used to treat pain and inflammation in rheumatoid arthritis and osteoarthritis, postoperative pain, acute pain including muscle and back pain, toothache and menstrual pain, as well as being prescribed for menorrhagia. There is evidence that supports the use of mefenamic acid for perimenstrual migraine headache prophylaxis, with treatment starting two days prior to the onset of flow or one day prior to the expected onset of the headache and continuing for the duration of menstruation. Mefenamic acid is recommended to be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parke-Davis
Parke-Davis is a subsidiary of the pharmaceutical company Pfizer. Although Parke, Davis & Co. is no longer an independent corporation, it was once America's oldest and largest drug maker, and played an important role in medical history. In 1970 Parke-Davis was acquired by Warner-Lambert, which in turn was acquired by Pfizer in 2000. History Parke, Davis and Company was founded in Detroit, Michigan by Dr. Samuel P. Duffield, a physician and pharmacist. In 1860, Dr. Duffield owned a small drugstore at the corner of Gratiot and Woodward Avenues. Dr. Duffield made a variety of pharmaceutical preparations, including Hoffman’s anodyne and mercurial ointment, but was overwhelmed by the operations of the business. Dr. Duffield and Hervey Coke Parke formed a partnership in October 1866, with George S. Davis becoming a third partner in 1867. Parke was a businessman looking for business opportunities and Davis, an ambitious man with skills in sales. Duffield withdrew in 1869 beca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generic Drug
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is equivalent in performance. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging. Although they may not be associated with a particular company, generic drugs are usually subject to government regulations in the countries in which they are dispensed. They are labeled with the name of the manufacturer and a generic non-proprietary name such as the United States Adopted Name (USAN) or International Nonproprietary Name (INN) of the drug. A generic drug must contain the same active ingredients as the original brand-name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AKR1C3
Aldo-keto reductase family 1 member C3 (AKR1C3), also known as 17β-hydroxysteroid dehydrogenase type 5 (17β-HSD5, HSD17B5) is a key steroidogenic enzyme that in humans is encoded by the ''AKR1C3'' gene. Function This gene encodes a member of the aldo/keto reductase superfamily, which consists of more than 40 known enzymes and proteins. These enzymes catalyze the conversion of aldehydes and ketones to their corresponding alcohols by utilizing NADH and/or NADPH as cofactors. The enzymes display overlapping but distinct substrate specificity. This enzyme catalyzes the reduction of prostaglandin D2, prostaglandin H2, and phenanthrenequinone, and the oxidation of prostaglandin F2α to prostaglandin D2. It is also capable of metabolizing estrogen and progesterone. AKR1C3 may play an important role in the development of allergic diseases such as asthma, and may also have a role in controlling cell growth and/or differentiation. This gene shares high sequence identity with three ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylation
In chemistry, hydroxylation can refer to: *(i) most commonly, hydroxylation describes a chemical process that introduces a hydroxyl group () into an organic compound. *(ii) the ''degree of hydroxylation'' refers to the number of OH groups in a molecule. The ''pattern of hydroxylation'' refers to the location of hydroxy groups on a molecule or material. Hydroxylation reactions Synthetic hydroxylations Installing hydroxyl groups into organic compounds can be effected by various metal catalysts. Many such catalysts are biomimetic, i.e. they are inspired by or intended to mimic enzymes such as cytochrome P450. Whereas many hydroxylations insert O atoms into bonds, some reactions ''add'' OH groups to unsaturated substrates. The Sharpless dihydroxylation is such a reaction: it converts alkenes into diols. The hydroxy groups are provided by hydrogen peroxide, which adds across the double bond of alkenes. Biological hydroxylation In biochemistry, hydroxylation reactions are often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |