|
Fludorex
Fludorex is a stimulant anorexic agent of the phenethylamine chemical class. Synthesis Grignard reaction between 3-Bromobenzotrifluoride 01-78-5(1) and 1,2-dibromo-1-methoxyethaneCID:13226400(2) leads to 1-(2-bromo-1-methoxyethyl)-3-(trifluoromethyl)benzeneCID:10589008(3). The reaction with methylamine Methylamine is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. Methylamine is sold as a solution in methanol, ... gives fludorex (4). See also The benzylamine is calleSK&F 39728-Ah1> References Stimulants Trifluoromethyl compounds Ethers Phenylethanolamine ethers Monoamine releasing agents {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fludorex Synthesis
Fludorex is a stimulant anorexic agent of the phenethylamine chemical class. Synthesis Grignard reaction between 3-Bromobenzotrifluoride 01-78-5(1) and 1,2-dibromo-1-methoxyethaneCID:13226400(2) leads to 1-(2-bromo-1-methoxyethyl)-3-(trifluoromethyl)benzeneCID:10589008(3). The reaction with methylamine Methylamine is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. Methylamine is sold as a solution in methanol, ... gives fludorex (4). See also The benzylamine is calleSK&F 39728-Ah1> References Stimulants Trifluoromethyl compounds Ethers Phenylethanolamine ethers Monoamine releasing agents {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stimulant
Stimulants (also often referred to as psychostimulants or colloquially as uppers) is an overarching term that covers many drugs including those that increase activity of the central nervous system and the body, drugs that are pleasurable and invigorating, or drugs that have Sympathomimetic drug, sympathomimetic effects. Stimulants are widely used throughout the world as prescription medicines as well as without a prescription (either legally or Prohibition (drugs), illicitly) as performance-enhancing substance, performance-enhancing or recreational drug use, recreational drugs. Among narcotics, stimulants produce a noticeable crash or ''Comedown (drugs), comedown'' at the end of their effects. The most frequently prescribed stimulants as of 2013 were lisdexamfetamine (Vyvanse), methylphenidate (Ritalin), and amphetamine (Adderall). It was estimated in 2015 that the percentage of the world population that had used cocaine during a year was 0.4%. For the category "amphetamines and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Phenethylamine
Substituted phenethylamines (or simply phenethylamines) are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents. The structural formula of any substituted phenethylamine contains a phenyl ring that is joined to an amino (NH) group via a two-carbon sidechain. Hence, any substituted phenethylamine can be classified according to the substitution of hydrogen (H) atoms on phenethylamine's phenyl ring, sidechain, or amino group with a specific group of atoms. Many substituted phenethylamines are psychoactive drugs which belong to a variety of different drug classes, including central nervous system stimulants (e.g., amphetamine), hallucinogens (e.g., dl- 2,5-dimethoxy-4-methylamphetamine DOM), entactogens (e.g., 3,4-methylenedioxyamphe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Class
Chemical classification systems attempt to classify elements or compounds according to certain chemical functional or structural properties. Whereas the structural properties are largely intrinsic, functional properties and the derived classifications depend to a certain degree on the type of chemical interaction partners on which the function is exerted. Sometimes other criteria like purely physical ones (e.g. molecular weight) or - on the other hand - functional properties above the chemical level are also used for building chemical taxonomies. Some systems mix the various levels, resulting in hierarchies where the domains are slightly confused, for example having structural and functional aspects end up on the same level. Whereas chemical function is closely dependent on chemical structure, the situation becomes more involved when e.g. pharmacological function is integrated, because the QSAR can usually not be directly computed from structural qualities. Physico-chemical cla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reaction
The Grignard reaction () is an organometallic chemical reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides ( Grignard reagent) is added to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds. The reaction of an organic halide with magnesium is ''not'' a Grignard reaction, but provides a Grignard reagent. : Grignard reactions and reagents were discovered by and are named after the French chemist François Auguste Victor Grignard (University of Nancy, France), who published it in 1900 and was awarded the 1912 Nobel Prize in Chemistry for this work. Reaction mechanism Because carbon is more electronegative than magnesium, the carbon attached to magnesium functions as a nucleophile and attacks the electrophilic carbon atom that is present within the polar bond of a carbonyl group. The addition of the Grignard reagent to the carbonyl typically proceeds through a six-membered ring transition state. Based on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylamine
Methylamine is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. Methylamine is sold as a solution in methanol, ethanol, tetrahydrofuran, or water, or as the anhydrous gas in pressurized metal containers. Industrially, methylamine is transported in its anhydrous form in pressurized railcars and tank trailers. It has a strong odor similar to rotten fish. Methylamine is used as a building block for the synthesis of numerous other commercially available compounds. Industrial production Methylamine is prepared commercially by the reaction of ammonia with methanol in the presence of an aluminosilicate catalyst. Dimethylamine and trimethylamine are co-produced; the reaction kinetics and reactant ratios determine the ratio of the three products. The product most favored by the reaction kinetics is trimethylamine. : In this way, an estimated 115,000 tons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stimulants
Stimulants (also often referred to as psychostimulants or colloquially as uppers) is an overarching term that covers many drugs including those that increase activity of the central nervous system and the body, drugs that are pleasurable and invigorating, or drugs that have Sympathomimetic drug, sympathomimetic effects. Stimulants are widely used throughout the world as prescription medicines as well as without a prescription (either legally or Prohibition (drugs), illicitly) as performance-enhancing substance, performance-enhancing or recreational drug use, recreational drugs. Among narcotics, stimulants produce a noticeable crash or ''Comedown (drugs), comedown'' at the end of their effects. The most frequently prescribed stimulants as of 2013 were lisdexamfetamine (Vyvanse), methylphenidate (Ritalin), and amphetamine (Adderall). It was estimated in 2015 that the percentage of the world population that had used cocaine during a year was 0.4%. For the category "amphetamines and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
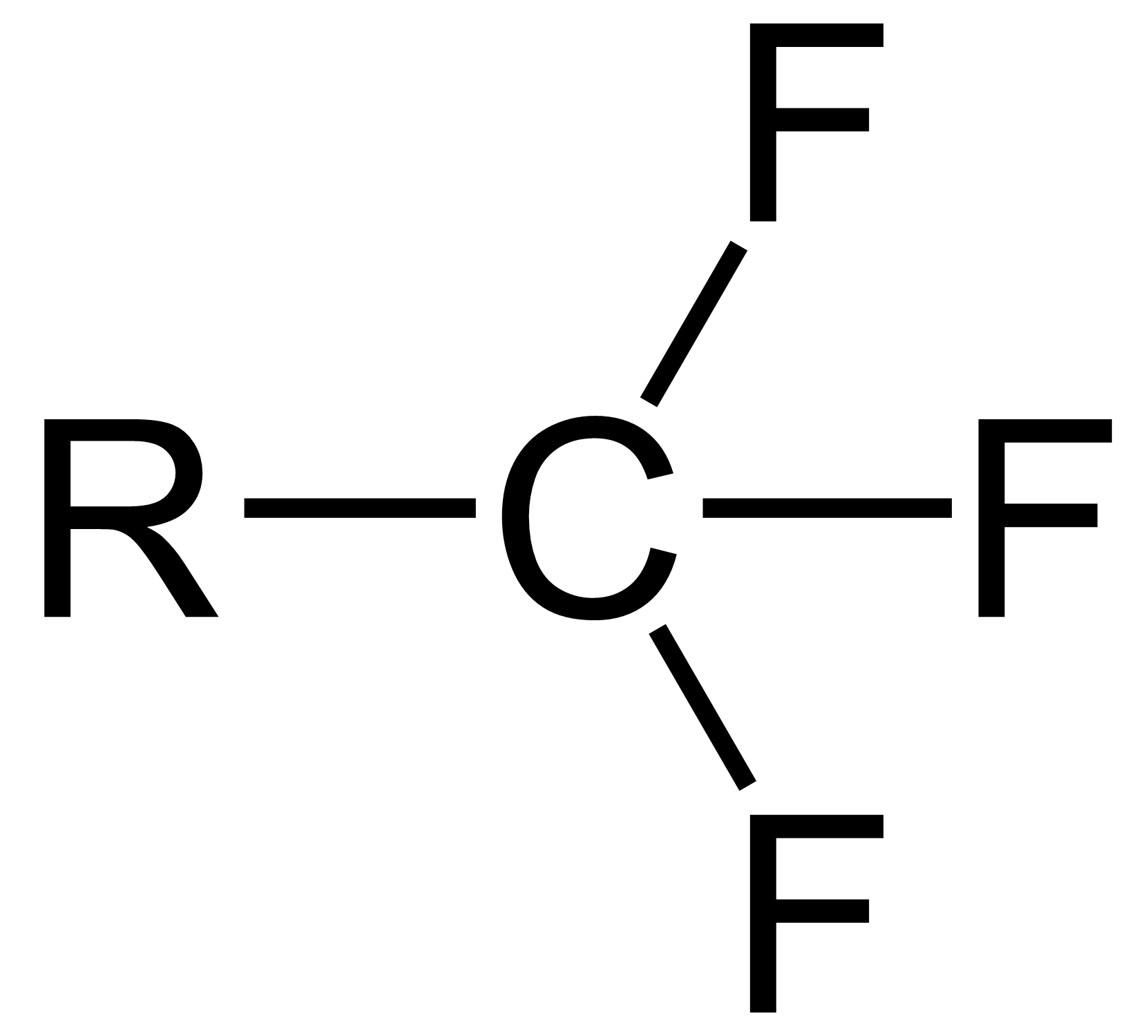
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethers
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylethanolamine Ethers
Phenylethanolamine (sometimes abbreviated PEOH), or β-hydroxyphenethylamine, is a trace amine with a structure similar to those of other trace phenethylamines as well as the catecholamine neurotransmitters dopamine, norepinephrine, and epinephrine. As an organic compound, phenylethanolamine is a β-hydroxylated phenethylamine that is also structurally related to a number of synthetic drugs in the substituted phenethylamine class. In common with these compounds, phenylethanolamine has strong cardiovascular activity and, under the name ''Apophedrin'', has been used as a drug to produce topical vasoconstriction.''The Merck Index, 10th Ed.'' (1983), p. 1051, Merck & Co., Rahway. In appearance, phenylethanolamine is a white solid. Phenylethanolamine is perhaps best known in the field of bioscience as part of the enzyme name " phenylethanolamine N-methyl transferase", referring to an enzyme which is responsible for the conversion of norepinephrine into epinephrine, as well as other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |