|
Fleming–Tamao Oxidation
The Fleming–Tamao oxidation, or Tamao–Kumada–Fleming oxidation, converts a carbon–silicon bond to a carbon–oxygen bond with a peroxy acid or hydrogen peroxide. Fleming–Tamao oxidation refers to two slightly different conditions developed concurrently in the early 1980s by the Kohei Tamao and Ian Fleming (chemist), Ian Fleming research groups. The reaction is stereospecific with retention of configuration at the carbon–silicon bond. This allows the silicon group to be used as a functional group, functional equivalent of the hydroxyl group. Another key feature of the silicon group is that it is relatively stable due to the presence of the silicon atom, and therefore can tolerate various reaction conditions that the hydroxyl group can not tolerate. Due to the stability of the silicon group, organosilicon compounds are useful in the total synthesis of complex natural products and pharmaceutical drugs. For instance, the Fleming–Tamao oxidation has been used to accompli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ian Fleming (chemist)
Ian Fleming (born 1935) is an England, English organic chemist, and an emeritus professor of the University of Cambridge, and an emeritus fellow of Pembroke College, Cambridge. He was the first to determine the full structure of chlorophyll (in 1967) and was involved in the development of the organic synthesis, synthesis of cyanocobalamin by Robert Burns Woodward. He has made major contributions to the use of organosilicon compounds for stereochemistry, stereospecific syntheses; reactions which have found application in the synthesis of natural compounds. He is also a prolific author, and has written a number of textbooks, encyclopedia chapters and influential review articles. Life and research Ian Fleming was born August 4, 1935, in Staffordshire and grew up in Stourbridge, Worcestershire. He received a Bachelor of Arts, B.A. in 1959 and a Doctor of Philosophy, Ph.D. in 1962, both from Pembroke College, Cambridge. His post-doctoral studies were done at Harvard University with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived , referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a free element in nature. Instead, it can be isolated from colourless soluble crystalline mineral halide Ionic salt, salts analogous to table salt, a property it shares with the other halogens. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its Bromine cycle, accumulation in the oceans. Commercially the element is easily extracted from brine evaporation ponds, mostly in the United States and Israel. The mass of bromine in the oce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallics
''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is organometallic and organometalloid chemistry. This peer-reviewed journal has an impact factor of 3.837 as reported by the 2021 Journal Citation Reports by Thomson Reuters. Since 2015 Paul Chirik is the editor-in-chief of ''Organometallics''. He is an American chemist and the Edwards S. Sanford Professor of Chemistry at Princeton University, and associate director for external partnerships of the Andlinger Center for Energy and the Environment. He writes about the catalysis of hydrocarbons. Past editors-in-chief are Dietmar Seyferth and John Gladysz. Retrieved on 2014-07-30. This journal is indexed in |
Basic (chemistry)
In chemistry, there are three definitions in common use of the word "base": '' Arrhenius bases'', '' Brønsted bases'', and ''Lewis bases''. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acidic
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Arrhenius acids. Brønsted and Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and react with bases and certain metals (like calcium) to form salts. The word ''acid'' is derived from the Latin , meaning 'sour'. An aqueous solution of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
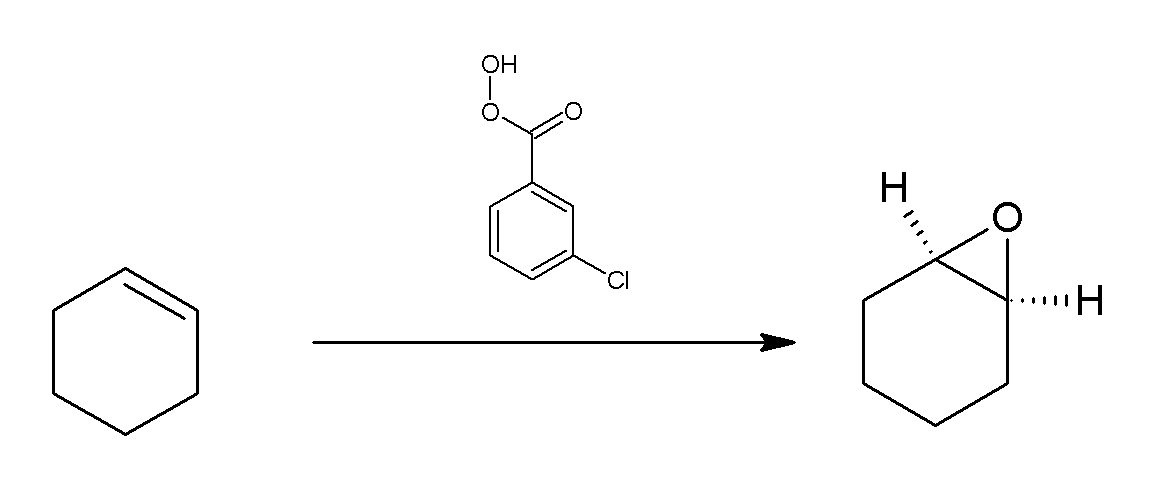
M-CPBA
''meta''-Chloroperoxybenzoic acid (mCPBA or ''m''CPBA) is a peroxycarboxylic acid. It is a white solid often used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material. Preparation and purification mCPBA can be prepared by reacting m-chlorobenzoyl chloride with a basic solution of hydrogen peroxide, followed by acidification. It is sold commercially as a shelf-stable mixture that is less than 72% mCPBA, with the balance made up of ''m''-chlorobenzoic acid (10%) and water. The peroxyacid can be purified by washing the commercial material with a sodium hydroxide and potassium phosphate solution buffered at pH = 7.5. Peroxyacids are generally slightly less acidic than their carboxylic acid counterparts, so the acid impurity can be extracted if the pH is carefully controlled. The purified material is reasonably sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemists
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of matter and its properties. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms. Chemists carefully measure substance proportions, chemical reaction rates, and other chemical properties. In Commonwealth English, pharmacists are often called chemists. Chemists use their knowledge to learn the composition and properties of unfamiliar substances, as well as to reproduce and synthesize large quantities of useful naturally occurring substances and create new artificial substances and useful processes. Chemists may specialize in any number of subdisciplines of chemistry. Materials scientists and metallurgists share much of the same education and skills wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylic Rearrangement
An allylic rearrangement or allylic shift is an organic reaction, organic chemical reaction in which reaction at a center Vicinal (chemistry), vicinal to a double bond causes the double bond to shift to an adjacent pair of atoms: It is encountered in both nucleophilic substitution, nucleophilic and electrophilic substitution, although it is usually Side reaction, suppressed relative to non-allylic substitution. For example, reaction of 1-chloro-2-butene with sodium hydroxide gives 2-buten-1-ol and 3-buten-2-ol: In the similar substitution of 1-chloro-3-methyl-2-butene, the secondary 2-methyl-3-buten-2-ol is produced in a yield of 85%, while that for the primary 3-methyl-2-buten-1-ol is 15%. Allylic shifts occur because the transition state is an Allyl group, allyl intermediate. In other respects they are similar to classical nucleophilic substitution, and admit both SN2 reaction, bimolecular and SN1 reaction, monomolecular mechanisms (respectively the SN2' and SN1'/SNi' substi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Alcohol
Allyl alcohol (IUPAC name: prop-2-en-1-ol) is an organic compound with the structural formula . Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols. Production Allyl alcohol is produced commercially by the Olin and Shell corporations through the hydrolysis of allyl chloride: : Allyl alcohol can also be made by the rearrangement of propylene oxide, a reaction that is catalyzed by potassium alum at high temperature. The advantage of this method relative to the allyl chloride route is that it does not generate salt. Also avoiding chloride-containing intermediates is the "acetoxylation" of propylene to allyl acetate: : Hydrolysis of this acetate gives allyl alcohol. In alternative fashion, propylene can be oxidized to acrole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silyl
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. Silylations are core methods for production of organosilicon chemistry. Silanization, while similar to silylation, usually refers to attachment of silyl groups to solids. Silyl groups are commonly used for: alcohol protection, enolate trapping, gas chromatography, electron-impact mass spectrometry (EI-MS), and coordinating with metal complexes. Protection Chemistry Protection Silylation is often used to protect alcohols, as well as amines, carboxylic acids, and terminal alkynes. The products after silylation, namely silyl ethers and silyl amines, are resilient toward basic conditions. Protection is typically done by reacting the functional group with a silyl halide by an SN2 reaction mechanism, typically in the presence of base. The protection mechanism begins with the base deprotonating the alcohol group. Next, the deprotonated alcohol group attacks the silyl atom of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |