|
Far-western Blot
The far-western blot, or far-western blotting, is a molecular biological method based on the technique of western blot to detect protein-protein interaction ''in vitro''. Whereas western blot uses an antibody probe to detect a protein of interest, far-western blot uses a non-antibody probe which can bind the protein of interest. Thus, whereas western blotting is used for the detection of certain proteins, far-western blotting is employed to detect protein/protein interactions. Method In conventional western blot, gel electrophoresis is used to separate proteins from a sample; these proteins are then transferred to a membrane in a 'blotting' step. In a western blot, specific proteins are then identified using an antibody probe. Far-western blot employs non-antibody proteins to probe the protein of interest on the blot. In this way, binding partners of the probe (or the blotted) protein may be identified. The probe protein is often produced in ''E. coli'' using an expression clonin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
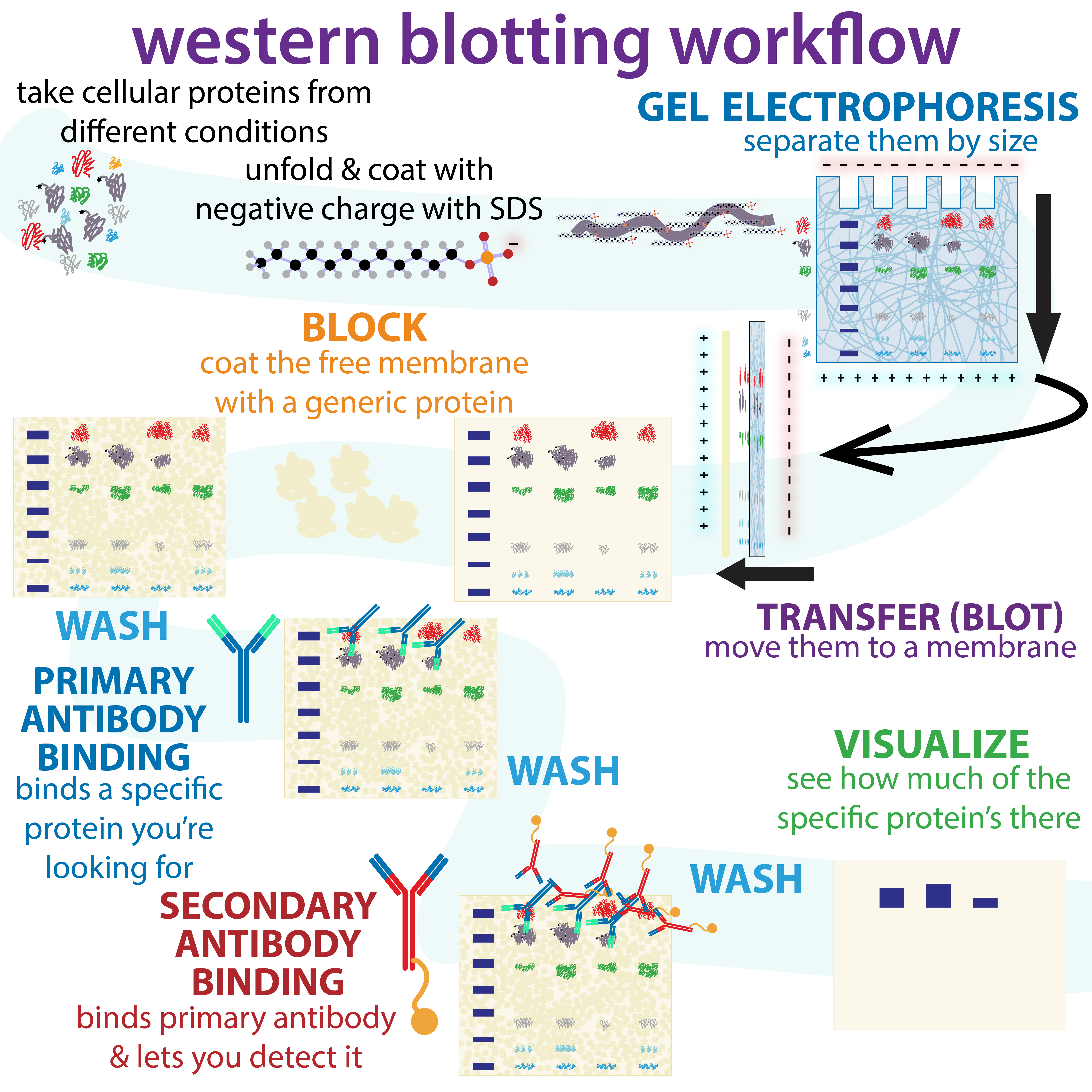
Western Blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination. Western blot technique uses three elements to achieve its task of separating a specific protein from a complex: separation by size, transfer of protein to a solid support, and marking target protein using a primary and secondary antibody to visualize. A synthetic or animal-derived antibody (known as the primary antibody) is created that recognizes and binds to a specific target protein. The electrophoresis membrane is washed in a solution containing the primary antibody, before excess antibody is washed off. A secondary antibody is added which recognizes and binds to the primary antibody ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blot (biology)
A blot, in molecular biology and genetics, is a method of transferring proteins, DNA or RNA onto a carrier (for example, a nitrocellulose, polyvinylidene fluoride or nylon membrane). In many instances, this is done after a gel electrophoresis, transferring the molecules from the gel onto the Blotting matrix, blotting membrane, and other times adding the samples directly onto the membrane. After the blotting, the transferred proteins, DNA or RNA are then visualized by colorant staining (for example, silver staining of proteins), autoradiographic visualization of radioactive tracer, radiolabelled molecules (performed before the blot), or specific labelling of some proteins or nucleic acids. The latter is done with antibody, antibodies or hybridization probes that bind only to some molecules of the blot and have an enzyme joined to them. After proper washing, this enzymatic activity (and so, the molecules we search in the blot) is visualized by incubation with proper reactive, renderin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gel Electrophoresis
Gel electrophoresis is a method for separation and analysis of biomacromolecules ( DNA, RNA, proteins, etc.) and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size (IEF agarose, essentially size independent) and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge. Nucleic acid molecules are separated by applying an electric field to move the negatively charged molecules through a matrix of agarose or other substances. Shorter molecules move faster and migrate farther than longer ones because shorter molecules migrate more easily through the pores of the gel. This phenomenon is called sieving. Proteins are separated by the charge in agarose because the pores of the gel are too small to sieve proteins. Gel electrophoresis can also be used for the separation of nanoparticles. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope (analogous to a lock) that is specific for one particular epitope (analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can ''tag'' a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly (for example, by blocking a part of a virus that is essential for its invasion). To allow the immune system to recognize millions of different antigens, the antigen-binding sites at both tips of the antibody come in an equally wide variety. In contrast, the remainder of the antibody is relatively constant. It only occurs in a few varia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Expression Cloning
Expression cloning is a technique in DNA cloning that uses expression vectors to generate a library of clones, with each clone expressing one protein. This ''expression library'' is then screened for the property of interest and clones of interest are recovered for further analysis. An example would be using an expression library to isolate genes that could confer antibiotic resistance. Expression vectors Expression vectors are a specialized type of cloning vector in which the transcriptional and translational signals needed for the regulation of the gene of interest are included in the cloning vector. The transcriptional and translational signals may be synthetically created to make the expression of the gene of interest easier to regulate. Purpose Usually the ultimate aim of expression cloning is to produce large quantities of specific proteins. To this end, a bacterial expression clone may include a ribosome binding site ( Shine-Dalgarno sequence) to enhance translation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity Tag
Protein tags are peptide sequences genetically grafted onto a recombinant protein. Tags are attached to proteins for various purposes. They can be added to either end of the target protein, so they are either C-terminus or N-terminus specific or are both C-terminus and N-terminus specific. Some tags are also inserted at sites within the protein of interest; they are known as internal tags. Affinity tags are appended to proteins so that they can be purified from their crude biological source using an affinity technique. Affinity tags include chitin binding protein (CBP), maltose binding protein (MBP), Strep-tag and glutathione-S-transferase (GST). The poly(His) tag is a widely used protein tag, which binds to matrices bearing immobilized metal ions. Solubilization tags are used, especially for recombinant proteins expressed in species such as '' E. coli'', to assist in the proper folding in proteins and keep them from aggregating in inclusion bodies. These tags include thior ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyhistidine-tag
A polyhistidine-tag is an amino acid motif in proteins that typically consists of at least six histidine (''His'') residues, often at the N- or C-terminus of the protein. It is also known as hexa histidine-tag, 6xHis-tag, His6 tag, by the US trademarked name HIS TAG (US Trademark serial number 74242707), and most commonly as His-Tag. The tag was invented by Roche, although the use of histidines and its vectors are distributed by Qiagen. Various purification kits for histidine-tagged proteins are available from Qiagen, Sigma-Aldrich Corporation, Sigma, Thermo Scientific, GE Healthcare, Macherey-NagelCube Biotech Clontech, Bio-Radand others. MK(HQ)6 may be used for enhanced expression in ''E. coli'' and tag removal. The total number of histidine residues may vary in the tag from as low as two, to as high as 10 or more His residues. N- or C-terminal His-tags may also be followed or preceded, respectively, by a suitable amino acid sequence that facilitates removal of the polyhistidine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FLAG-tag
FLAG-tag, or FLAG octapeptide, or FLAG epitope, is a peptide protein tag that can be added to a protein using recombinant DNA technology, having the sequence DYKDDDDK (where D=aspartic acid, Y=tyrosine, and K=lysine). It is one of the most specific tags and it is an artificial antigen to which specific, high affinity monoclonal antibodies have been developed and hence can be used for protein purification by affinity chromatography and also can be used for locating proteins within living cells. FLAG-tag has been used to separate recombinant, overexpressed protein from wild-type protein expressed by the host organism. FLAG-tag can also be used in the isolation of protein complexes with multiple subunits, because FLAG-tag's mild purification procedure tends not to disrupt such complexes. FLAG-tag-based purification has been used to obtain proteins of sufficient purity and quality to carry out 3D structure determination by x-ray crystallography. A FLAG-tag can be used in many different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Biology Techniques
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |