|
Fadogia Homblei
''Fadogia homblei'' is a 60 cm-tall erect perennial sub-Saharan shrublet with subterranean stems producing unbranched annual shoots, and is one of some 47 species of ''Fadogia'' in the family Rubiaceae. It occurs in Angola, Tanzania, Malawi, Mozambique, Zambia, Zimbabwe, and in Limpopo and Gauteng Provinces in South Africa. This species has leaves in whorls of 3-5. The leaves are elliptic or lanceolate, shiny above, with greyish-white papillose hairs on the undersurface. Fragrant flowers are in 3-5-flowered whorls arising from the leaf nodes, and are creamy yellow to bright yellow in colour. The fruit is spherical, crowned with the persistent calyx limb, initially green, but turning black when ripe, and is edible. Browsing of this species has long been known to cause 'gousiekte' ("quick disease"), a cardiotoxicosis of ruminants marked by heart failure four to eight weeks after ingestion of certain species of ''Vangueria'', ''Pavetta'', and ''Fadogia'', and is thought to be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
De Wild
De Wild may refer to: * De Wild Family, a Dutch family of art professionals * Ruud de Wild (born 1969), a Dutch radio host * ''De Wild.'', taxonomic author abbreviation for Émile Auguste Joseph De Wildeman (1866–1947), Belgian botanist See also * De Wilde, Dutch surname * De Wildt De Wildt is town with a railway station, police station and post office in North West province of South Africa, 40 km west-north-west of Pretoria. History It was named after the Dutch engineer Mauritz Edgar de Wildt (1855–1907), who in 190 ..., town and wildlife centre in South Africa named after Mauritz Edgar de Wildt {{surname Dutch-language surnames ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fadogia
''Fadogia'' is a genus of flowering plants in the family Rubiaceae. The genera ''Rytigynia'' and ''Fadogia'' form a strongly supported clade but neither of these genera is monophyletic. Distribution ''Fadogia'' is found in Tropical Africa. '' F. cienkowskii'' and '' F. tetraquetra'' have the largest distribution and occur from Guinea to the Transvaal province. '' F. ancylantha'' and '' F. erythrophloea'' are also found in many African countries, but they don't occur so far south. The countries with the highest number of species are Angola, Democratic Republic of the Congo, Zambia, Tanzania, and Central African Republic. Bacterial leaf symbiosis Endophytic bacteria are housed in the intercellular space of the leaf mesophyll tissue. The presence of these bacteria can only be microscopically ascertained. The bacteria are identified as ''Burkholderia'', which is a genus that is also found in the leaves of other Rubiaceae species. The hypothesis is that these endophytic bacteria pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uracil
Uracil () (symbol U or Ura) is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine (T). Uracil is a demethylated form of thymine. Uracil is a common and naturally occurring pyrimidine derivative. The name "uracil" was coined in 1885 by the German chemist Robert Behrend, who was attempting to synthesize derivatives of uric acid. Originally discovered in 1900 by Alberto Ascoli, it was isolated by hydrolysis of yeast nuclein; it was also found in bovine thymus and spleen, herring sperm, and wheat germ. It is a planar, unsaturated compound that has the ability to absorb light. Based on 12C/13C isotopic ratios of organic compounds found in the Murchison meteorite, it is believed that uracil, xanthine, and related molecules can also be formed extraterrestrially. Data from the Cassini mission, orbiting in the Saturn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinoresinol
Pinoresinol is a tetrahydrofuran lignan found in ''Styrax sp.'', ''Forsythia suspensa, and in Forsythia koreana''. It is also found in the caterpillar of the cabbage butterfly, ''Pieris rapae'' where it serves as a defence against ants. In food, it is found in sesame seed, in ''Brassica'' vegetables and in olive oil. Pinoresinol has also been found to be toxic to larvae of the milkweed bug Oncopeltus fasciatus and of the haematophagous insect Rhodnius prolixus, which is a vector of chagas disease. Currently, pinoresinol is isolated from plants with low efficiency and low yield. Biosynthesis A first dirigent protein was discovered in ''Forsythia intermedia''. This protein has been found to direct the stereoselective biosynthesis of (+)-pinoresinol from coniferyl alcohol monomers. Recently, a second, enantiocomplementary dirigent protein was identified in ''Arabidopsis thaliana'', which directs enantioselective synthesis of (-)-pinoresinol. Pharmacology Pinoresinol inhibits ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stigmasterol
Stigmasterol – a plant sterol (''phytosterol'') – is among the most abundant of plant sterols, having a major function to maintain the structure and physiology of cell membranes. In the European Union, it is a food additive listed with E number E499, and may be used in food manufacturing to increase the phytosterol content, potentially lowering the levels of LDL cholesterol. Discovery Once called ''Wulzen factor'' in the mid-20th century, stigmasterol was discovered by the University of California physiologist Rosalind Wulzen (born 1886). Natural occurrences Stigmasterol is an unsaturated phytosterol occurring in the plant fats or oils of numerous plants, such as soybean, calabar bean, and rape seed, and in herbs used in herbalism practices, including the Chinese herbs ''Ophiopogon japonicus'' (Mai men dong), in ''Mirabilis jalapa''. Stigmasterol is a constituent of various vegetables, legumes, nuts, seeds, and unpasteurized milk. Pasteurization will inactivate stig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
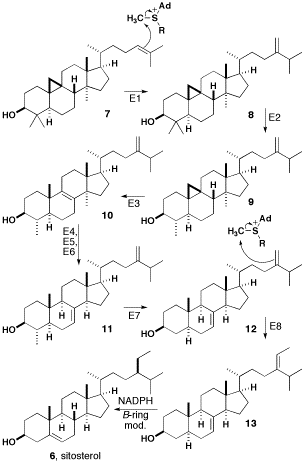
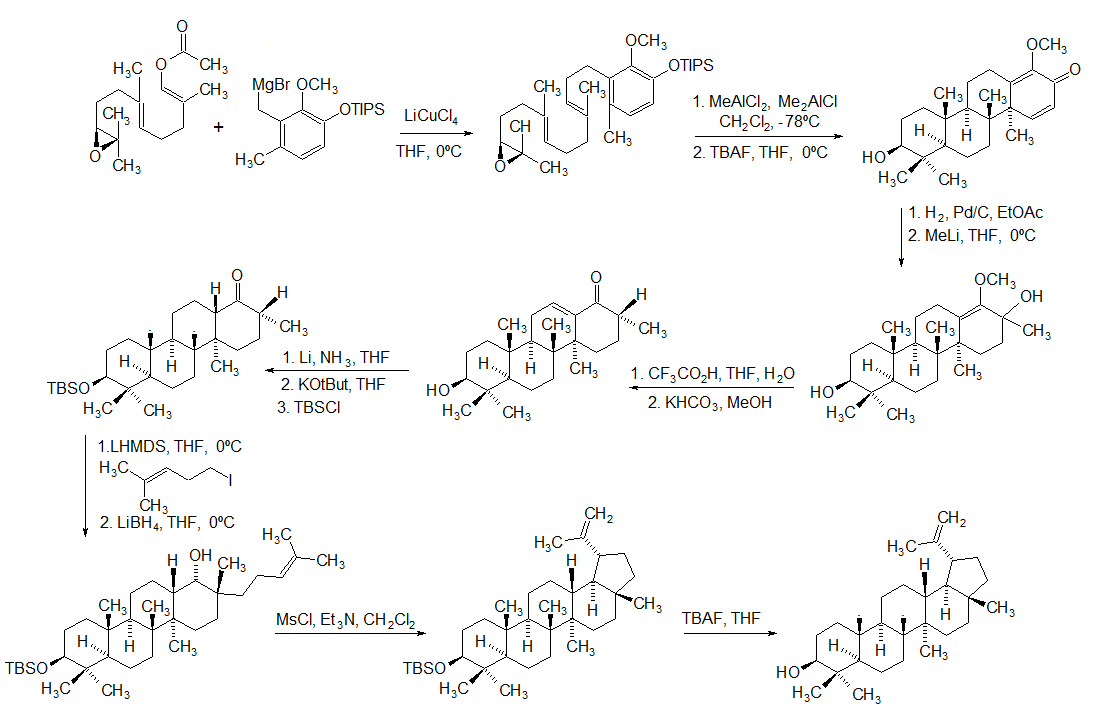
Sitosterol
β-sitosterol (beta-sitosterol) is one of several phytosterols (plant sterols) with chemical structures similar to that of cholesterol. It is a white, waxy powder with a characteristic odor, and is one of the components of the food additive E499. Phytosterols are hydrophobic and soluble in alcohols. Natural occurrences and food β-sitosterol is widely distributed in the plant kingdom. It is found in vegetable oil, nuts, avocados, and derived prepared foods such as salad dressings. Human research β-sitosterol is being studied for its potential to reduce benign prostatic hyperplasia (BPH) and blood cholesterol levels. Genetic disorder While plant sterols are usually beneficial, there is a rare autosomal recessive genetic disorder phytosterolemia which causes over-absorption of phytosterols. Precursor of anabolic steroid boldenone Being a steroid, β-sitosterol is a precursor of anabolic steroid boldenone. Boldenone undecylenate is commonly used in veterinary medicine to induce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Betulinic Acid
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase. It is found in the bark of several species of plants, principally the white birch (''Betula pubescens'') from which it gets its name, but also the ber tree (''Ziziphus mauritiana''), selfheal (''Prunella vulgaris''), the tropical carnivorous plants '' Triphyophyllum peltatum'' and ''Ancistrocladus heyneanus'', '' Diospyros leucomelas'', a member of the persimmon family, '' Tetracera boiviniana'', the jambul (''Syzygium formosanum''), flowering quince (''Pseudocydonia sinensis'', former ''Chaenomeles sinensis KOEHNE''), (''Chaenomeles sinensis KOEHNE'' is now named ''Pseudocydonia sinensis'') rosemary, and ''Pulsatilla chinensis''. Antitumor activity In 1995, betulinic acid was reported as a selective inhibitor of human melanoma. Then i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lupeol
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity. Natural occurrences Lupeol is found in a variety of plants, including mango, '' Acacia visco'' and '' Abronia villosa''. It is also found in dandelion coffee. Lupeol is present as a major component in ''Camellia japonica'' leaf. Total synthesis The first total synthesis of lupeol was reported by Gilbert Stork ''et al''. In 2009, Surendra and Corey reported a more efficient and enantioselective total synthesis of lupeol, starting from (''1E,5E'')-8- ''2S'')-3,3-dimethyloxiran-2-yl2,6-dimethylocta-1,5-dienyl acetate by use of a polycyclization. Biosynthesis Lupeol is produced by several organisms from squalene epoxide. Dammarane and baccharane skeletons are formed as intermediates. The reactions are catalyzed by the enzyme lupeol synthase. A recent study on the metabolomics of ''Camellia japonica'' leaf revealed t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quercetin
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods. Occurrence Quercetin is a flavonoid widely distributed in nature. The name has been used since 1857, and is derived from ''quercetum'' (oak forest), after the oak genus ''Quercus''. It is a naturally occurring polar auxin transport inhibitor. Quercetin is one of the most abundant dietary flavonoids, with an average daily consumption of 25–50 milligrams. In red onions, higher concentrations of quercetin occur in the outermost rings and in the part closest to the root, the latter being the part of the plant with the highest concentration. One study found that organically grown tomatoes had 79% more quercetin than non-organically grown fruit. Quercetin is presen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luteolin
Luteolin is a flavone, a type of flavonoid, with a yellow crystalline appearance. Luteolin is the principal yellow dye compound that is obtained from the plant ''Reseda luteola'', which has been used as a source of the dye since at least the first millennium B.C. Luteolin was first isolated in pure form, and named, in 1829 by the French chemist Michel Eugène Chevreul. The luteolin empirical formula was determined by the Austrian chemists Heinrich Hlasiwetz and Leopold Pfaundler in 1864. In 1896, the English chemist Arthur George Perkin proposed the correct structure for luteolin. Perkin's proposed structure for luteolin was confirmed in 1900 when the Polish-Swiss chemist Stanislaw Kostanecki (1860–1910) and his students A. Różycki and J. Tambor synthesized luteolin. Natural occurrences Luteolin is most often found in leaves, but it is also seen in rinds, barks, clover blossom, and ragweed pollen. It has also been isolated from the aromatic flowering plant, ''Salvia t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scopoletin
Scopoletin is a coumarin. It found in the root of plants in the genus ''Scopolia'' such as ''Scopolia carniolica'' and ''Scopolia japonica'', in chicory, in '' Artemisia scoparia'', in the roots and leaves of stinging nettle (''Urtica dioica''), in the passion flower, in '' Brunfelsia'', in ''Viburnum prunifolium'', in ''Solanum nigrum'', in ''Datura metel'', in '' Mallotus resinosus'', or and in '' Kleinhovia hospita''. It can also be found in fenugreek, vinegar, some whiskies or in dandelion coffee. A similar coumarin is scoparone. Scopoletin is highly fluorescent when dissolved in DMSO or water and is regularly used as a fluorimetric assay for the detection of hydrogen peroxide in conjunction with horseradish peroxidase. When oxidized, its fluorescence is strongly suppressed. Chemistry Biosynthesis Like most phenylpropanoids, the biosynthetic precursor to scopoletin acid is 4-coumaroyl-CoA. Scopoletin is derived from 1,2-benzopyrones which is the core structure of couma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyamine
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature. Natural polyamines Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine spermidine and the tetraamine spermine. They are structurally and biosynthetically related to the diamines putrescine and cadaverine. Polyamine metabolism is regulated by the activity of the enzyme ornithine decarboxylase (ODC). Polyamines are found in high concentrations in the mammalian brain. File:Spermidine-2D-skeletal.svg, spermidine File:Spermine.svg, spermine Synthetic polyamines Several synthetic polyamines are used in chemical industry and the research laboratory. They are mainly of interest as additives to motor oil and as co-rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |