|
Erepsin
Erepsin is a mixture of enzymes contained in a protein fraction found in the intestinal juices that digest peptones into amino acids. It is produced and secreted by the intestinal glands in the ileum and the pancreas, but it is also found widely in other cells. It is, however, a term now rarely used in scientific literature as more precise terms are preferred. History Erepsin was discovered at the beginning of the twentieth century by German physiologist Otto Cohnheim (1873-1953) who found a substance that breaks down peptones into amino acid in the intestines. He termed this hypothetical protease in his protein extract "erepsin" in 1901, derived from a Greek word meaning "I break down" (έρείπω). His discovery was significant as it overturned the previous "hypothesis of resynthesis" which proposed that proteins get broken down into peptones from which proteins may then be resynthesized, and helped establish the idea of free amino acids instead of peptones being the building ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digestion
Digestion is the breakdown of large insoluble food molecules into small water-soluble food molecules so that they can be absorbed into the watery blood plasma. In certain organisms, these smaller substances are absorbed through the small intestine into the blood stream. Digestion is a form of catabolism that is often divided into two processes based on how food is broken down: mechanical and chemical digestion. The term mechanical digestion refers to the physical breakdown of large pieces of food into smaller pieces which can subsequently be accessed by digestive enzymes. Mechanical digestion takes place in the mouth through mastication and in the small intestine through segmentation contractions. In chemical digestion, enzymes break down food into the small molecules the body can use. In the human digestive system, food enters the mouth and mechanical digestion of the food starts by the action of mastication (chewing), a form of mechanical digestion, and the wetting contact o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dipeptidases
Dipeptidases are enzymes secreted by enterocytes into the small intestine. Dipeptidases hydrolyze bound pairs of amino acids, called dipeptides. Dipeptidases are secreted onto the brush border of the villi in the small intestine, where they cleave dipeptides into their two component amino acids prior to absorption. They are also found within the enterocytes themselves, performing cytosolic digestion of absorbed dipeptides. Dipeptidases are exopeptidases, classified under EC number 3.4.13. See also * Membrane dipeptidase Membrane dipeptidase (, ''renal dipeptidase'', ''dehydropeptidase I (DPH I)'', ''dipeptidase'', ''aminodipeptidase'', ''dipeptide hydrolase'', ''dipeptidyl hydrolase'', ''nonspecific dipeptidase'', ''glycosyl-phosphatidylinositol-anchored renal di ... References External links * Enzymes EC 3.4.13 {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exopeptidase
An exopeptidase is any peptidase that catalyzes the cleavage of the terminal (or the penultimate) peptide bond; the process releases a single amino acid, dipeptide or a tripeptide from the peptide chain. Depending on whether the amino acid is released from the amino or the carboxy terminal (N-terminus or C-terminus), an exopeptidase is further classified as an aminopeptidase or a carboxypeptidase, respectively. Thus, an aminopeptidase, an enzyme in the brush border of the small intestine, will cleave a single amino acid from the amino terminal, whereas carboxypeptidase, which is a digestive enzyme present in pancreatic juice, will cleave a single amino acid from the carboxylic end of the peptide. Some examples of exopeptidases include: * Carboxypeptidase A - cleaves C-terminal Phe, Tyr, Trp, or Leu * Carboxypeptidase B - cleaves C-terminal Lys or Arg * Aminopeptidase - cleaves any N-terminal amino acid * Prolinase - cleaves N-terminal Pro from dipeptides * Prolidase - cleaves C-te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prolidase
Xaa-Pro dipeptidase, also known as prolidase, is an enzyme that in humans is encoded by the ''PEPD'' gene. Function Xaa-Pro dipeptidase is a cytosolic dipeptidase that hydrolyzes dipeptides with proline or hydroxyproline at the carboxy terminus (but not Pro-Pro). It is important in collagen metabolism because of the high levels of imino acids. Mutations at the PEPD locus cause prolidase deficiency. This is characterised by Iminodipeptidurea, skin ulcers, mental retardation and recurrent infections. Structure Prolidases fall under a subclass of metallopeptidases that involve binuclear active site metal clusters. This metal cluster facilitates catalysis by serving as a substrate binding site, activating nucleophiles, and stabilizing the transition state. Furthermore, prolidases are classified under a smaller family called “pita-bread” enzymes, which cleave amido-, imine, imido-, and amidine, amidino- containing bonds. The “pita-bread” fold, containing a metal center ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prolinase
Cytosolic non-specific dipeptidase () also known as carnosine dipeptidase 2 is an enzyme that in humans is encoded by the ''CNDP2'' gene. This enzyme catalyses the following chemical reaction : Hydrolysis of dipeptides, preferentially hydrophobic dipeptides including prolyl amino acids This zinc enzyme has broad specificity. Nomenclature Cytosolic non-specific dipeptidase is also known as * N2-beta-alanylarginine dipeptidase * glycyl-glycine dipeptidase * glycyl-leucine dipeptidase * iminodipeptidase * peptidase A * Pro-X dipeptidase * prolinase * prolyl dipeptidase * prolylglycine dipeptidase * L-prolylglycine dipeptidase * diglycinase * Gly-Leu hydrolase * glycyl-L-leucine dipeptidase * glycyl-L-leucine hydrolase * glycyl-L-leucine peptidase * L-amino-acyl-L-amino-acid hydrolase * glycylleucine peptidase * glycylleucine hydrolase * glycylleucine dipeptide hydrolase * non-specific dipeptidase * human cytosolic non-specific dipeptidase Dipeptidase is a type of peptidase, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
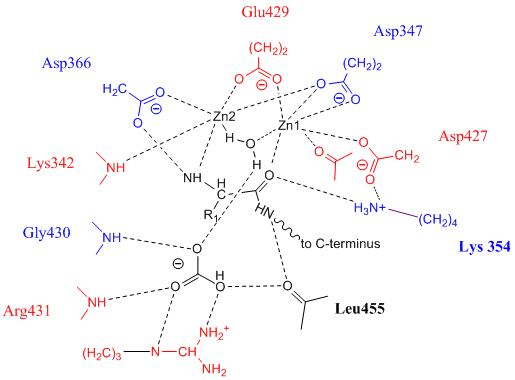
Leucyl Aminopeptidase
Leucyl aminopeptidases (, ''leucine aminopeptidase'', ''LAPs'', ''leucyl peptidase'', ''peptidase S'', ''cytosol aminopeptidase'', ''cathepsin III'', ''L-leucine aminopeptidase'', ''leucinaminopeptidase'', ''leucinamide aminopeptidase'', ''FTBL proteins'', ''proteinates FTBL'', ''aminopeptidase II'', ''aminopeptidase III'', ''aminopeptidase I'') are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, ''Escherichia coli'' (''E. coli'') LAP (also known as PepA or XerB), and the solanaceous-specific acidic LAP (LAP-A) in tomato (''Solanum lycopersicum''). Enzyme description, structure, and active site The active sites in PepA and in bovine lens LAP have been found to be similar. Shown in the picture below is the proposed model for the active site of LAP-A in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxypeptidases
A carboxypeptidase ( EC number 3.4.16 - 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed. Functions Initial studies on carboxypeptidases focused on pancreatic carboxypeptidases A1, A2, and B in the digestion of food. Most carboxypeptidases are not, however, involved in catabolism. Instead they help to mature proteins, for example Post-translational modification. They also regulate biological processes, such as the biosynthesis of neuroendocrine peptides such as insulin requires a carboxypeptidase. Carboxypeptidases also function in blood clotting, growth factor production, wound healing, reproduction, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminopeptidases
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the amino terminus (N-terminus) of proteins or peptides (exopeptidases). They are widely distributed throughout the animal and plant kingdoms and are found in many subcellular organelles, in cytosol, and as membrane components. Aminopeptidases are used in essential cellular functions. Many, but not all, of these peptidases are zinc metalloenzymes. Some aminopeptidases are monomeric, and others are assemblies of relatively high mass (50 kDa) subunits. cDNA sequences are available for several aminopeptidases and a crystal structure of the open state of human endoplasmic reticulum Aminopeptidase 1 ERAP1 is presented here. Amino acid sequences determined directly or deduced from cDNAs indicate some amino acid sequence homologies in organisms as diverse as ''Escherichia coli'' and mammals, particularly in catalytically important residues or in residues involved in metal ion binding. One important aminopeptidas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxypeptidase
A carboxypeptidase ( EC number 3.4.16 - 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed. Functions Initial studies on carboxypeptidases focused on pancreatic carboxypeptidases A1, A2, and B in the digestion of food. Most carboxypeptidases are not, however, involved in catabolism. Instead they help to mature proteins, for example Post-translational modification. They also regulate biological processes, such as the biosynthesis of neuroendocrine peptides such as insulin requires a carboxypeptidase. Carboxypeptidases also function in blood clotting, growth factor production, wound healing, reproduction, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dipeptidase
Dipeptidases are enzymes secreted by enterocytes into the small intestine. Dipeptidases hydrolyze bound pairs of amino acids, called dipeptides. Dipeptidases are secreted onto the brush border of the villi in the small intestine, where they cleave dipeptides into their two component amino acids prior to absorption. They are also found within the enterocytes themselves, performing cytosolic digestion of absorbed dipeptides. Dipeptidases are exopeptidases, classified under EC number 3.4.13. See also * Membrane dipeptidase Membrane dipeptidase (, ''renal dipeptidase'', ''dehydropeptidase I (DPH I)'', ''dipeptidase'', ''aminodipeptidase'', ''dipeptide hydrolase'', ''dipeptidyl hydrolase'', ''nonspecific dipeptidase'', ''glycosyl-phosphatidylinositol-anchored renal di ... References External links * Enzymes EC 3.4.13 {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |