|
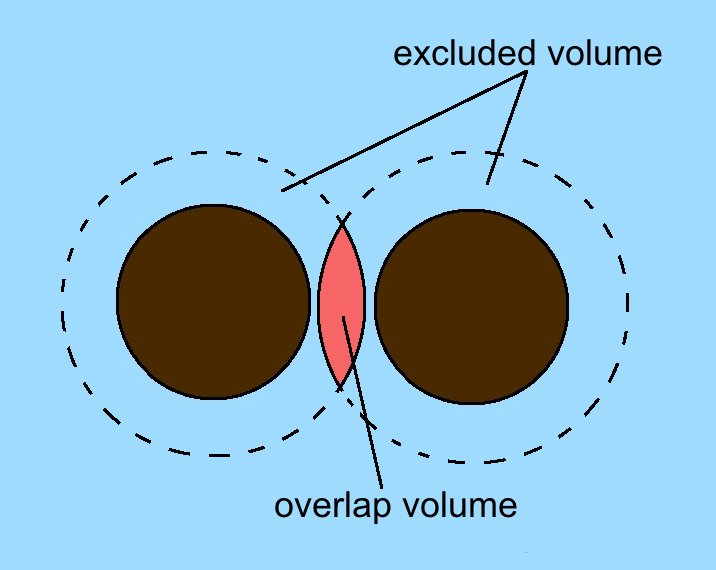
Excluded Volume
The concept of excluded volume was introduced by Werner Kuhn in 1934 and applied to polymer molecules shortly thereafter by Paul Flory. Excluded volume gives rise to depletion forces. In liquid state theory In liquid state theory, the 'excluded volume' of a molecule is the volume that is inaccessible to other molecules in the system as a result of the presence of the first molecule. The excluded volume of a hard sphere is eight times its volume—however, for a two-molecule system, this volume is distributed among the two particles, giving the conventional result of four times the volume; this is an important quantity in the Van der Waals equation of state. The calculation of the excluded volume for particles with non-spherical shapes is usually difficult, since it depends on the relative orientation of the particles. The distance of closest approach of hard ellipses and their excluded area has been recently considered. In polymer science In polymer science, excluded volume ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Werner Kuhn (chemist)
Werner Kuhn (February 6, 1899 – August 27, 1963) was a Swiss physical chemist who developed the first model of the viscosity of polymer solutions using statistical mechanics.Werner Kuhn - Encyclopædia Britannica He is known for being the first to apply : : to the modeling of molecules, i.e. the "rubber band model", |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Flory
Paul John Flory (June 19, 1910 – September 9, 1985) was an American chemist and Nobel laureate who was known for his work in the field of polymers, or macromolecules. He was a leading pioneer in understanding the behavior of polymers in solution, and won the Nobel Prize in Chemistry in 1974 "for his fundamental achievements, both theoretical and experimental, in the physical chemistry of macromolecules". Biography Personal life Flory was born in Sterling, Illinois, on June 19, 1910. He was raised by Ezra Flory and Nee Martha Brumbaugh. His father worked as a clergyman-educator, and his mother was a school teacher. He first gained his interest in science from Carl W Holl, who was a professor in chemistry. Holl was employed in Indiana at Manchester College as a chemistry professor. In 1936, he married Emily Catherine Tabor. He and Emily had three children together; Susan Springer, Melinda Groom and Paul John Flory jr. They also had five grandchildren. All of his children purs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depletion Force
A depletion force is an effective attractive force that arises between large colloidal particles that are suspended in a dilute solution of ''depletants'', which are smaller solutes that are preferentially excluded from the vicinity of the large particles. One of the earliest reports of depletion forces that lead to particle coagulation is that of Bondy, who observed the separation or "creaming" of rubber latex upon addition of polymer depletant molecules (sodium alginate) to solution. More generally, depletants can include polymers, micelles, osmolytes, ink, mud, or paint dispersed in a continuous phase. Depletion forces are often regarded as entropic forces, as was first explained by the established Asakura–Oosawa model. In this theory the depletion force arises from an increase in osmotic pressure of the surrounding solution when colloidal particles get close enough such that the excluded cosolutes (depletants) cannot fit in between them. Because the particles were consider ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Equation
In chemistry and thermodynamics, the Van der Waals equation (or Van der Waals equation of state) is an equation of state which extends the ideal gas law to include the effects of interaction between molecules of a gas, as well as accounting for the finite size of the molecules. The ideal gas law treats gas molecules as point particles that interact with their containers but not each other, meaning they neither take up space nor change kinetic energy during collisions (i.e. all collisions are perfectly elastic). The ideal gas law states that the volume ''V'' occupied by ''n'' moles of any gas has a pressure ''P'' at temperature ''T'' given by the following relationship, where ''R'' is the gas constant: :PV=nRT To account for the volume occupied by real gas molecules, the Van der Waals equation replaces V/n in the ideal gas law with (V_m-b), where ''Vm'' is the molar volume of the gas and ''b'' is the volume occupied by the molecules of one mole: :P(V_m - b)=R T The second m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distance Of Closest Approach Of Ellipses And Ellipsoids
The distance of closest approach of two objects is the distance between their centers when they are externally tangent. The objects may be geometric shapes or Particle, physical particles with well-defined boundaries. The distance of closest approach is sometimes referred to as the contact distance. For the simplest objects, spheres, the distance of closest approach is simply the sum of their radii. For non-spherical objects, the distance of closest approach is a function of the orientation of the objects, and its calculation can be difficult. The maximum packing density of hard particles, an important problem of ongoing interest, depends on their distance of closest approach. The interactions of particles typically depend on their separation, and the distance of closest approach plays an important role in determining the behavior of condensed matter systems. Excluded volume The excluded volume of particles (the volume excluded to the centers of other particles due to the pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Chain
Ideal may refer to: Philosophy * Ideal (ethics), values that one actively pursues as goals * Platonic ideal, a philosophical idea of trueness of form, associated with Plato Mathematics * Ideal (ring theory), special subsets of a ring considered in abstract algebra * Ideal, special subsets of a semigroup * Ideal (order theory), special kind of lower sets of an order * Ideal (set theory), a collection of sets regarded as "small" or "negligible" * Ideal (Lie algebra), a particular subset in a Lie algebra * Ideal point, a boundary point in hyperbolic geometry * Ideal triangle, a triangle in hyperbolic geometry whose vertices are ideal points Science * Ideal chain, in science, the simplest model describing a polymer * Ideal gas law, in physics, governing the pressure of an ideal gas * Ideal transformer, an electrical transformer having zero resistance and perfect magnetic threading * Ideal final result, in TRIZ methodology, the best possible solution * Thought experiment, sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theta Solvent
In a polymer solution, a theta solvent (or θ solvent) is a solvent in which polymer coils act like ideal chains, assuming exactly their random walk coil dimensions. Therefore, the Mark–Houwink equation exponent is 1/2 in a theta solvent. Thermodynamically, the excess chemical potential of mixing between a polymer and a theta solvent is zero. Physical interpretation The conformation assumed by a polymer chain in dilute solution can be modeled as a random walk of monomer subunits using a freely jointed chain model. However, this model does not account for steric effects. Real polymer coils are more closely represented by a self-avoiding walk because conformations in which different chain segments occupy the same space are not physically possible. This excluded volume effect causes the polymer to expand. Chain conformation is also affected by solvent quality. The intermolecular interactions between polymer chain segments and coordinated solvent molecules have an associated ener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distance Of Closest Approach
The distance of closest approach of two objects is the distance between their centers when they are externally tangent. The objects may be geometric shapes or physical particles with well-defined boundaries. The distance of closest approach is sometimes referred to as the contact distance. For the simplest objects, spheres, the distance of closest approach is simply the sum of their radii. For non-spherical objects, the distance of closest approach is a function of the orientation of the objects, and its calculation can be difficult. The maximum packing density of hard particles, an important problem of ongoing interest, depends on their distance of closest approach. The interactions of particles typically depend on their separation, and the distance of closest approach plays an important role in determining the behavior of condensed matter systems. Excluded volume The excluded volume of particles (the volume excluded to the centers of other particles due to the presence of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mayer F-function
The Mayer f-function is an auxiliary function that often appears in the series expansion of thermodynamic quantities related to classical many-particle systems.Donald Allan McQuarrie, ''Statistical Mechanics'' (HarperCollins, 1976), page 228 It is named after chemist and physicist Joseph Edward Mayer. Definition Consider a system of classical particles interacting through a pair-wise potential :V(\mathbf,\mathbf) where the bold labels \mathbf and \mathbf denote the continuous degrees of freedom associated with the particles, e.g., :\mathbf=\mathbf_i for spherically symmetric particles and :\mathbf=(\mathbf_i,\Omega_i) for rigid non-spherical particles where \mathbf denotes position and \Omega the orientation parametrized e.g. by Euler angles. The Mayer f-function is then defined as :f(\mathbf,\mathbf)=e^-1 where \beta=(k_T)^ the inverse absolute temperature in units of (Temperature times the Boltzmann constant k_)−1 . See also *Virial coefficient *Cluster expansion *Excluded vol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Physics
Polymer physics is the field of physics that studies polymers, their fluctuations, mechanical properties, as well as the kinetics of reactions involving degradation and polymerisation of polymers and monomers respectively.P. Flory, ''Principles of Polymer Chemistry'', Cornell University Press, 1953. .Pierre Gilles De Gennes, ''Scaling Concepts in Polymer Physics'' CORNELL UNIVERSITY PRESS Ithaca and London, 1979M. Doi and S. F. Edwards, ''The Theory of Polymer Dynamics'' Oxford University Inc NY, 1986 While it focuses on the perspective of condensed matter physics, polymer physics is originally a branch of statistical physics. Polymer physics and polymer chemistry are also related with the field of polymer science, where this is considered the applicative part of polymers. Polymers are large molecules and thus are very complicated for solving using a deterministic method. Yet, statistical approaches can yield results and are often pertinent, since large polymers (i.e., polymers wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |