|
Europium(III) Phosphate
Europium(III) phosphate is one of the phosphates of europium, with the chemical formula of EuPO4. Other phosphates include europium(II) phosphate (Eu3(PO4)2) and europium(II,III) phosphate (Eu3Eu(PO4)3). Preparation Europium phosphate can be produced by the sol-gel method of europium(III) oxide. First, europium(III) oxide was dissolved in an equimolar amount of nitric acid, and then an excess of 10% phosphoric acid was added. The process also requires the addition of ammonia to adjust the pH to 4 and form a gel, which is then washed with water and heated to 1200 °C for a day. Properties Europium(III) phosphate is isotypic to CePO4 and crystallizes in the monazite structure type, in the space group ''P''21/n (no. 14, position 2) with the lattice parameters ''a'' = 668.13(10), ''b'' = 686.18(9), ''c'' = 634.91(8) pm and β = 103.96(1)° with four formula units per unit cell. Its heat capacity is 111.5 J·K−1·mol−1 at 298.15 K, and its bulk modulus The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoric Acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non- volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Removal of all three ions gives the phosphate ion . Removal of one or two protons gives dihydrogen phosphate ion , and the hydrogen phosphate ion , respectively. Phosphoric acid forms esters, called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Production Phosphoric acid is produced industrially by one of two routes, wet processes and dry. We ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bulk Modulus
The bulk modulus (K or B) of a substance is a measure of how resistant to compression the substance is. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume. Other moduli describe the material's response (strain) to other kinds of stress: the shear modulus describes the response to shear stress, and Young's modulus describes the response to normal (lengthwise stretching) stress. For a fluid, only the bulk modulus is meaningful. For a complex anisotropic solid such as wood or paper, these three moduli do not contain enough information to describe its behaviour, and one must use the full generalized Hooke's law. The reciprocal of the bulk modulus at fixed temperature is called the isothermal compressibility. Definition The bulk modulus K (which is usually positive) can be formally defined by the equation :K=-V\frac , where P is pressure, V is the initial volume of the substance, and dP/dV denotes the derivative of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its thermal mass. Definition Basic definition The heat capacity of an object, denoted by C, is the limit : C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably depending on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strukturbericht Designation
In crystallography, a Strukturbericht designation or Strukturbericht type is a system of detailed crystal structure classification by analogy to another known structure. The designations were intended to be comprehensive but are mainly used as supplement to space group crystal structures designations, especially historically. Each Strukturbericht designation is described by a single space group, but the designation includes additional information about the positions of the individual atoms, rather than just the symmetry of the crystal structure. While Strukturbericht symbols exist for many of the earliest observed and most common crystal structures, the system is not comprehensive, and is no longer being updated. Modern databases such as Inorganic Crystal Structure Database index thousands of structure types directly by the prototype compound (i.e. "the NaCl structure" instead of "the B1 structure"). These are essentially equivalent to the old Stukturbericht designations. History T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium-dominant member of the group. It occurs usually in small isolated crystals. It has a hardness of 5.0 to 5.5 on the Mohs scale of mineral hardness and is relatively dense, about 4.6 to 5.7 g/cm3. There are five different most common species of monazite, depending on the relative amounts of the rare earth elements in the mineral: * monazite-(Ce), ( Ce, La, Nd, Th) PO4 (the most common member), * monazite-(La), (La,Ce,Nd)PO4, * monazite-(Nd), (Nd,La,Ce)PO4, * monazite-(Sm), ( Sm, Gd,Ce,Th)PO4, * monazite-(Pr), ( Pr,Ce,Nd,Th)PO4. The elements in parentheses are listed in the order of their relative proportion within the mineral: lanthanum is the most common rare-earth element in monazite-(La), and so forth. Silica (SiO2) is present in trace a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
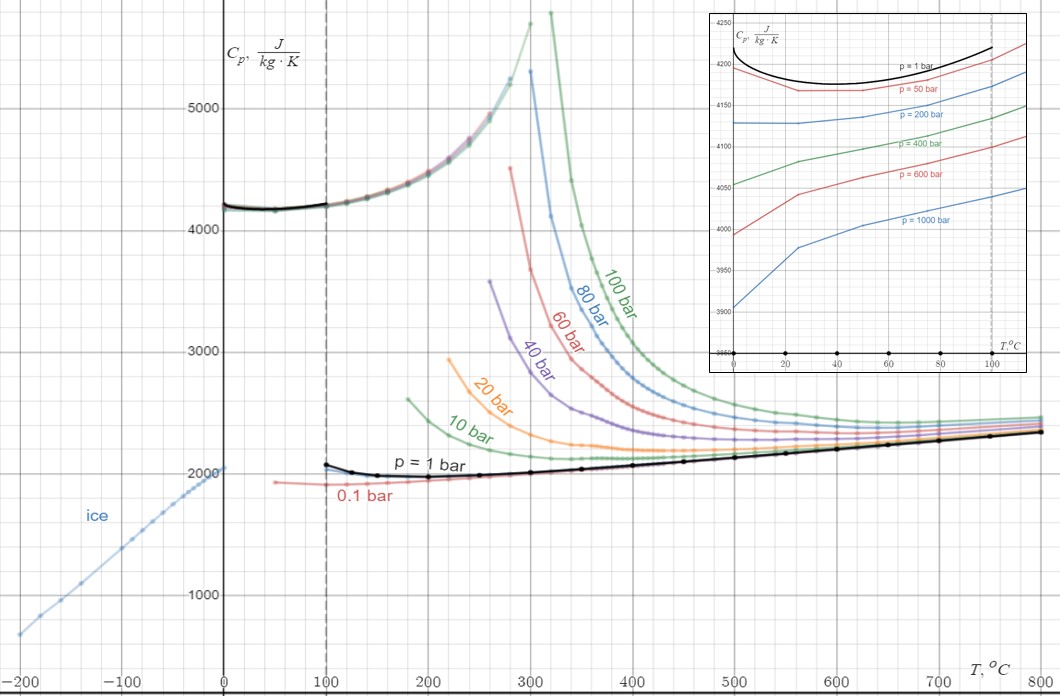
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Europium(III) Oxide
Europium(III) oxide (Eu2O3), is a chemical compound of europium and oxygen. It is widely used as a red or blue phosphor in television sets and fluorescent lamps, and as an activator for yttrium-based phosphors. It is also an agent for the manufacture of fluorescent glass. Europium fluorescence is used in the anti-counterfeiting phosphors in Euro banknotes. Europium oxide has two common structures: Monoclinic ( mS30, space group ''C''2/''m'', No. 12) and cubic ( cI80, space group ''I''a, No. 206). The cubic structure is similar to that of manganese(III) oxide. It may be formed by ignition of europium Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ... metal. It can react with acids to form the corresponding europium(III) salts. Gallery File:Tl2O3structure.jpg, Cubic Eu2O3 File:Gd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-3D-balls.png)