|
Erythrohydrobupropion
Erythrohydrobupropion (developmental code names BW 287, BW 17U) is a substituted amphetamine derivative—specifically a β-hydroxyamphetamine—and a minor active metabolite of the antidepressant drug bupropion (Wellbutrin). Bupropion is a norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor negative allosteric modulator, with its metabolites contributing substantially to its activities. Erythrohydrobupropion exists as two isomers, (1''R'',2''S'')-erythrohydrobupropion and (1''S'',2''R'')-erythrohydrobupropion. Other metabolites of bupropion include hydroxybupropion and threohydrobupropion. Information on the pharmacological actions of erythrohydrobupropion is scarce. In any case, it is about 20% as pharmacologically potent as bupropion and in the range of 20 to 50% as potent as bupropion in mouse models of depression. It circulates at similar concentrations as bupropion during bupropion therapy. Conversely, two other metabolites, hydroxybupropio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threohydrobupropion
Threohydrobupropion (developmental code names BW 494, BW A494U) is a substituted amphetamine derivative—specifically a β-hydroxyamphetamine—and a major active metabolite of the antidepressant drug bupropion (Wellbutrin). Bupropion is a norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor negative allosteric modulator, with its metabolites contributing substantially to its activities. Threohydrobupropion exists as two isomers, (1''R'',2''R'')-threohydrobupropion and (1''S'',2''S'')-threohydrobupropion. Other metabolites of bupropion include hydroxybupropion and erythrohydrobupropion. Information on the pharmacological actions of threohydrobupropion is scarce. In any case, it is about 20% as pharmacologically potent as bupropion and in the range of 20 to 50% as potent as bupropion in mouse models of depression. Moreover, threohydrobupropion has been reported to weakly inhibit the reuptake of norepinephrine, dopamine, and serotonin with rat o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
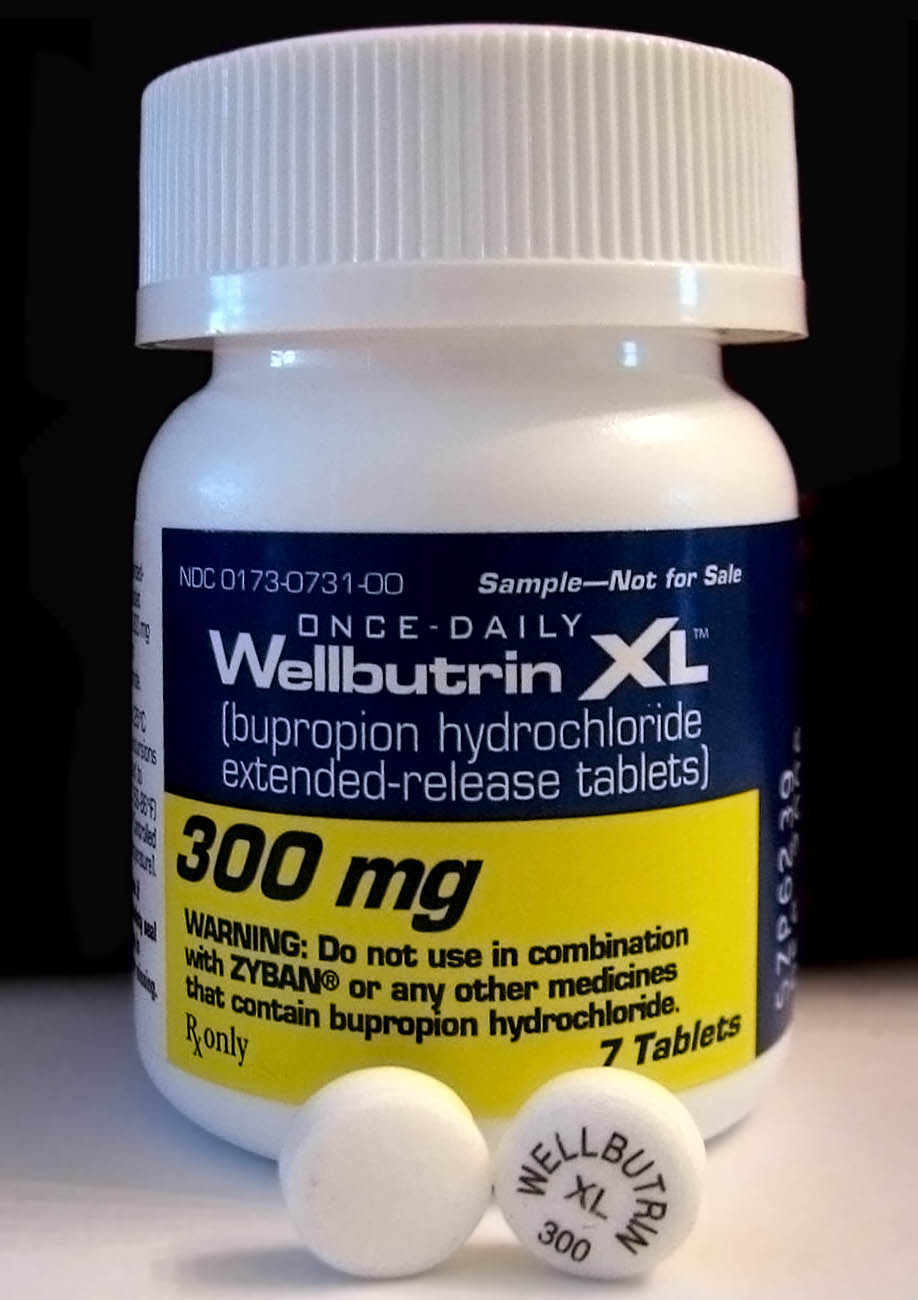
Bupropion
Bupropion, sold under the brand names Wellbutrin and Zyban among others, is an atypical antidepressant primarily used to treat major depressive disorder and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction; it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion does, however, carry a much higher risk of seizure than many other antidepressants and extreme caution must be taken in patients with a history of seizure disorder. Common adverse effects of bupropion with the greatest difference from placebo are dry mouth, nausea, constipation, insomnia, anxiety, tremor, and excessive sweating. Raised blood pressure is notable. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxybupropion
Hydroxybupropion (code name BW 306U), or 6-hydroxybupropion, is the major active metabolite of the antidepressant and smoking cessation drug bupropion. It is formed from bupropion by the liver enzyme CYP2B6 during first-pass metabolism. With oral bupropion treatment, hydroxybupropion is present in plasma at area under the curve concentrations that are as many as 16–20 times greater than those of bupropion itself, demonstrating extensive conversion of bupropion into hydroxybupropion in humans. As such, hydroxybupropion is likely to play a very important role in the effects of oral bupropion, which could accurately be thought of as functioning largely as a prodrug to hydroxybupropion. Other metabolites of bupropion besides hydroxybupropion include threohydrobupropion and erythrohydrobupropion. Pharmacology Pharmacodynamics Compared to bupropion, hydroxybupropion is similar in its potency as a norepinephrine reuptake inhibitor ( IC50 = 1.7 μM), but is substantially weaker a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate (biochemistry)
Biotransformation is the biochemical modification of one chemical compound or a mixture of chemical compounds. Biotransformations can be conducted with whole cells, their lysates, or purified enzymes. Increasingly, biotransformations are effected with purified enzymes. Major industries and life-saving technologies depend on biotransformations. Advantages and disadvantages Compared to the conventional production of chemicals, biotransformations are often attractive because their selectivities can be high, limiting the coproduction of undesirable coproducts. Generally operating under mild temperatures and pressures in aqueous solutions, many biotransformations are "green". The catalysts, i.e. the enzymes, are amenable to improvement by genetic manipulation. Biotechnology usually is restrained by substrate scope. Petrochemicals for example are often not amenable to biotransformations, especially on the scale required for some applications, e.g. fuels. Biotransformations can be s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucuronide
A glucuronide, also known as glucuronoside, is any substance produced by linking glucuronic acid to another substance via a glycosidic bond. The glucuronides belong to the glycosides. Glucuronidation, the conversion of chemical compounds to glucuronides, is a method that animals use to assist in the excretion of toxic substances, drugs or other substances that cannot be used as an energy source. Glucuronic acid is attached via a glycosidic bond to the substance, and the resulting glucuronide, which has a much higher water solubility than the original substance, is eventually excreted by the kidney The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; bloo ...s. Enzymes that cleave the glycosidic bond of a glucuronide are called glucuronidases. Examples * Miquelianin ( Quercetin 3-O-glucur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Estabrook, Cooper, and Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones. CYP enzymes have been identified in all kingdoms of life: animals, plants, fungi, protists, bacteria, and archaea, as well as in viruses. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. , more than 300,000 distinct CYP proteins are known. CYPs are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism. Metabolic reactions may be categorized as '' catabolic'' – the ''breaking down'' of compounds (for example, of glucose to pyruvate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Reductase
In enzymology, a carbonyl reductase (NADPH) () is an enzyme that catalyzes the chemical reaction R-CO-R' + NADPH + H+ \rightleftharpoons :R-CHOH-R' + NADP+ Thus, the two products of this enzyme are R-CHOH-R' and NADP+, whereas its 3 substrates are R-CO-R', NADPH, and H+. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is secondary-alcohol:NADP+ oxidoreductase. Other names in common use include aldehyde reductase 1, prostaglandin 9-ketoreductase, xenobiotic ketone reductase, NADPH-dependent carbonyl reductase, ALR3, carbonyl reductase, nonspecific NADPH-dependent carbonyl reductase, aldehyde reductase 1, and carbonyl reductase (NADPH). This enzyme participates in arachidonic acid metabolism, and has recently been shown to catabolize S-Nitrosoglutathione, as a means to degrade NO in an NADPH-dependent manner. Structural studies As of late 200 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldo-keto Reductase
The aldo-keto reductase family is a family of proteins that are subdivided into 16 categories; these include a number of related monomeric NADPH-dependent oxidoreductases, such as aldehyde reductase, aldose reductase, prostaglandin F synthase, xylose reductase, rho crystallin, and many others. Structure All possess a similar structure, with a beta-alpha-beta fold characteristic of nucleotide binding proteins. The fold comprises a parallel beta-8/alpha-8-barrel, which contains a novel NADP-binding motif. The binding site is located in a large, deep, elliptical pocket in the C-terminal end of the beta sheet, the substrate being bound in an extended conformation. The hydrophobic nature of the pocket favours aromatic and apolar substrates over highly polar ones. Binding of the NADPH coenzyme causes a massive conformational change, reorienting a loop, effectively locking the coenzyme in place. This binding is more similar to FAD- than to NAD(P)-binding oxidoreductases. Examples S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered reta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |