|
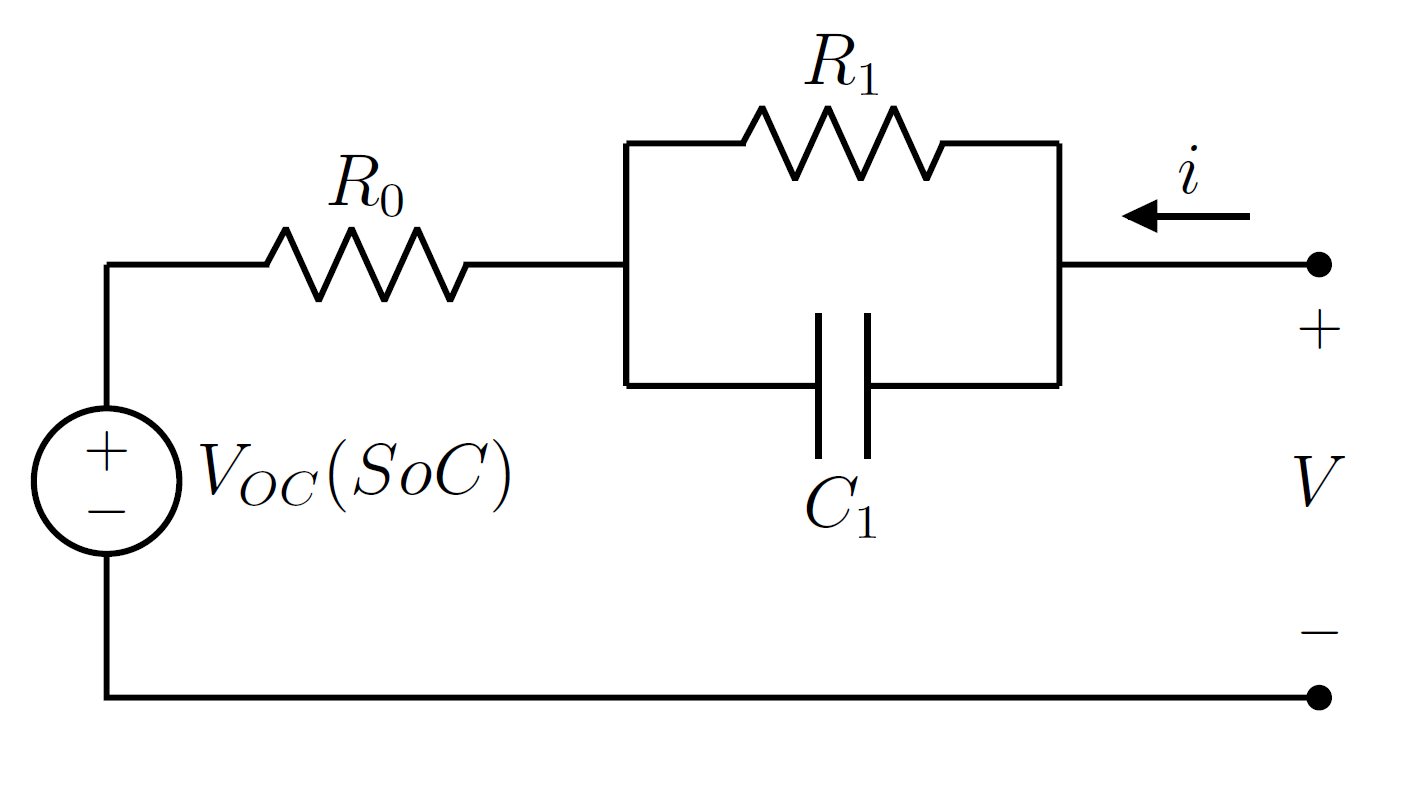
Equivalent Circuit Model For Li-ion Cells
The equivalent circuit model (ECM) is a common lumped-element model for Lithium-ion battery cells. The ECM Simulation, simulates the terminal voltage dynamics of a Li-ion cell through an Equivalent circuit, equivalent electrical network composed passive elements, such as resistors and capacitors, and a Voltage source, voltage generator. The ECM is widely employed in several application fields, including Computer simulation, computerized simulation, because of its simplicity, its low computational demand, its ease of characterization, and its structural flexibility. These features make the ECM suitable for real-time Battery management system, Battery Management System (BMS) tasks like state of charge (SoC) estimation, State of Health (SoH) monitoring and battery thermal management. Model structure The equivalent-circuit model is used to simulate the voltage at the cell terminals when an electric current is applied to discharge or recharge it. The most common circuital representat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lumped-element Model
The lumped-element model (also called lumped-parameter model, or lumped-component model) is a simplified representation of a physical system or circuit that assumes all components are concentrated at a single point and their behavior can be described by idealized mathematical models. The lumped-element model simplifies the system or circuit behavior description into a topology. It is useful in electrical systems (including electronics), mechanical multibody systems, heat transfer, acoustics, etc. This is in contrast to distributed parameter systems or models in which the behaviour is distributed spatially and cannot be considered as localized into discrete entities. The simplification reduces the state space of the system to a finite dimension, and the partial differential equations (PDEs) of the continuous (infinite-dimensional) time and space model of the physical system into ordinary differential equations (ODEs) with a finite number of parameters. Electrical systems ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Resistance
In electrical engineering, a practical electric power source which is a linear circuit may, according to Thévenin's theorem, be represented as an ideal voltage source in series with an impedance. This impedance is termed the internal resistance of the source. When the power source delivers current, the measured voltage output is lower than the no- load voltage; the difference is the voltage drop (the product of current and resistance) caused by the internal resistance. The concept of internal resistance applies to all kinds of electrical sources and is useful for analyzing many types of circuits. Battery A battery may be modeled as a voltage source in series with a resistance. These types of models are known as equivalent circuit models. Another common model being physiochemical models that are physical in nature involving concentrations and reaction rates. In practice, the internal resistance of a battery is dependent on its size, state of charge, chemical properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solvent like water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes. Michael Faraday coined the term "" in 1833; the word recalls the Greek ἤλεκτρον (, "amber") and ὁδός (, "path, way"). The electrophore, invented by Johan Wilcke in 1762, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Cobalt Oxide
Lithium cobalt oxide, sometimes called lithium cobaltateA. L. Emelina, M. A. Bykov, M. L. Kovba, B. M. Senyavin, E. V. Golubina (2011), "Thermochemical properties of lithium cobaltate". ''Russian Journal of Physical Chemistry'', volume 85, issue 3, pages 357–363; or lithium cobaltite,Ondřej Jankovský, Jan Kovařík, Jindřich Leitner, Květoslav Růžička, David Sedmidubský (2016) "Thermodynamic properties of stoichiometric lithium cobaltite LiCoO2". ''Thermochimica Acta'', volume 634, pages 26-30. is a chemical compound with formula . The cobalt atoms are formally in the +3 oxidation state, hence the IUPAC name lithium cobalt(III) oxide. Lithium cobalt oxide is a dark blue or bluish-gray crystalline solid,LinYi Gelon New Battery Materials Co., Ltd"Lithium Cobalt Oxide (LiCoO2) for lithium ion battery " Catalog entry, accessed on 2018-04-10, and is commonly used in the positive electrodes of lithium-ion batteries especially in handheld electronics. Structure The stru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Nickel Cobalt Aluminium Oxides
The lithium nickel cobalt aluminium oxides (abbreviated as Li-NCA, LNCA, or NCA) are a group of mixed metal oxides. Some of them are important due to their application in lithium-ion batteries. NCAs are used as active material in the positive electrode (which is the cathode when the battery is discharged). NCAs are composed of the cations of the chemical elements lithium, nickel, cobalt and aluminium. The compounds of this class have a general formula LiNi''x''Co''y''Al''z''O2 with ''x'' + ''y'' + ''z'' = 1. In case of the NCA comprising batteries currently available on the market, which are also used in electric cars and electric appliances, ''x'' ≈ 0.84, and the voltage of those batteries is between 3.6 V and 4.0 V, at a nominal voltage of 3.6 V or 3.7 V. A version of the oxides currently in use in 2019 is LiNi0.84Co0.12Al0.04O2. Properties of NCA The usable charge storage capacity of NCA is about 180 to 200 m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Nickel Manganese Cobalt Oxides
Lithium nickel manganese cobalt oxides (abbreviated NMC, Li-NMC, LNMC, or NCM) are mixed metal oxides of lithium, nickel, manganese and cobalt with the general formula LiNi''x''Mn''y''Co''1-x-y''O2. These materials are commonly used in lithium-ion batteries for mobile devices and electric vehicles, acting as the positively charged cathode. There is a particular interest in optimizing NMC for electric vehicle applications because of the material's high energy density and operating voltage. Reducing the cobalt content in NMC is also a current target, due to metal's high cost. Furthermore, an increased nickel content provides more capacity within the stable operation window. Structure NMC materials have layered structures similar to the individual metal oxide compound lithium cobalt oxide (LiCoO2). Lithium ions intercalate between the layers upon discharging, remaining between the lattice planes until the battery gets charged, at which point the lithium de-intercalates and moves ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Iron Phosphate Battery
The lithium iron phosphate battery ( battery) or LFP battery (''lithium ferrophosphate'') is a type of lithium-ion battery using lithium iron phosphate () as the cathode material, and a graphitic carbon electrode with a metallic backing as the anode. Because of their low cost, high safety, low toxicity, long cycle life and other factors, LFP batteries are finding a number of roles in vehicle use, utility-scale stationary applications, and backup power. LFP batteries are cobalt-free. As of September 2022, LFP type battery market share for EVs reached 31%, and of that, 68% were from EV makers Tesla and BYD alone. Chinese manufacturers currently hold a near-monopoly of LFP battery type production. With patents having started to expire in 2022 and the increased demand for cheaper EV batteries, LFP type production is expected to rise further and surpass lithium nickel manganese cobalt oxides (NMC) type batteries. By 2024, the LFP world market was estimated at $11-17 billion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current describes the direction in which positive charges move. Electrons, which are the carriers of current in most electrical systems, have a negative electrical charge, so the movement of electrons is ''opposite'' to that of the conventional current flow: this means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current (the flow of positive charges) in a circuit is opposite to the direction of electron flow, so (negatively charged) electrons flow from the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "+" is the cathode (while discharging). In both a galvanic cell and an electrolytic cell, the anode is the electrode at which the oxidation reaction occurs. In a galvanic cell the anode is the wire or plate having excess negative charge as a result of the oxidation reaction. In an electrolytic cell, the anode is the wire or plate upon which excess positive charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonlinear System
In mathematics and science, a nonlinear system (or a non-linear system) is a system in which the change of the output is not proportional to the change of the input. Nonlinear problems are of interest to engineers, biologists, physicists, mathematicians, and many other scientists since most systems are inherently nonlinear in nature. Nonlinear dynamical systems, describing changes in variables over time, may appear chaotic, unpredictable, or counterintuitive, contrasting with much simpler linear systems. Typically, the behavior of a nonlinear system is described in mathematics by a nonlinear system of equations, which is a set of simultaneous equations in which the unknowns (or the unknown functions in the case of differential equations) appear as variables of a polynomial of degree higher than one or in the argument of a function which is not a polynomial of degree one. In other words, in a nonlinear system of equations, the equation(s) to be solved cannot be written as a lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |