|
Epelsiban
Epelsiban ( INN, USAN, code name GSK-557,296-B) is an orally bioavailable drug which acts as a selective and potent oxytocin receptor antagonist ( Ki = 0.13 nM). It was initially developed by GlaxoSmithKline (GSK) for the treatment of premature ejaculation in men and then as an agent to enhance embryo or blastocyst implantation in women undergoing embryo or blastocyst transfer associated with ''in vitro'' fertilization ( IVF)., and was also investigated for use in the treatment of adenomyosis. Discovery and Design Screening the GSK compound collection and various libraries identified 2,5-diketopiperazines (2,5-DKPs) exemplified by 1 as novel and selective antagonists at the human oxytocin receptor (OTR). The lead, 1, showed potency of Ki = 300nM as a mixture of isomers in the amide side-chain. Initial structure–activity relationship (SAR) studies led to the semi-rigid and chirally pure 2,5-DKP 2 (Ki = 4nM), with ''cis'' disposed substituents at C-3 and C-6 and the ''R'' si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
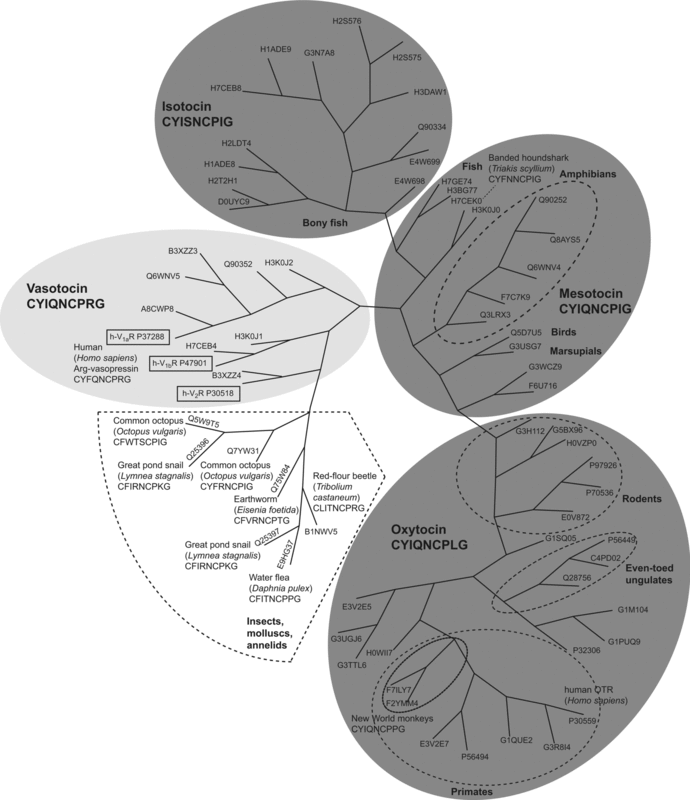
Oxytocin Receptor
The oxytocin receptor, also known as OXTR, is a protein which functions as receptor for the hormone and neurotransmitter oxytocin. In humans, the oxytocin receptor is encoded by the ''OXTR'' gene which has been localized to human chromosome 3p25. Function and location The OXTR protein belongs to the G-protein coupled receptor family, specifically Gq, and acts as a receptor for oxytocin. Its activity is mediated by G proteins that activate several different second messenger systems. Oxytocin receptors are expressed by the myoepithelial cells of the mammary gland, and in both the myometrium and endometrium of the uterus at the end of pregnancy. The oxytocin-oxytocin receptor system plays an important role as an inducer of uterine contractions during parturition and of milk ejection. OXTR is also associated with the central nervous system. The gene is believed to play a major role in social, cognitive, and emotional behavior. A decrease in OXTR expression by methylation of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retosiban
Retosiban also known as GSK-221,149-A is an oral drug which acts as an oxytocin receptor antagonist. It is being developed by GlaxoSmithKline for the treatment of preterm labour. Retosiban has high affinity for the oxytocin receptor (Ki = 0.65 nM) and has greater than 1400-fold selectivity over the related vasopressin receptors Mechanism of action Retosiban is a competitive oxytocin receptor antagonist which blocks the oxytocin-mediated contraction of the uterine smooth muscle in the female uterus that occurs during the initiation of preterm labour. This has been used to prevent preterm labour and premature birth. Pharmacology Retosiban has been shown to be an effective tocolytic. By intravenous and oral administration it produces a dose-dependent decrease in oxytocin-induced uterine contractions in non-pregnant female rats. In late-term pregnant rats it significantly reduces spontaneous uterine contractions in a dose-dependent manner by intravenous administration. In humans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Besylate
Benzenesulfonic acid (conjugate base benzenesulfonate) is an organosulfur compound with the formula C6 H6 O3 S. It is the simplest aromatic sulfonic acid. It forms white deliquescent sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in benzene and insoluble in nonpolar solvents like diethyl ether. It is often stored in the form of alkali metal salts. Its aqueous solution is strongly acidic. Preparation Benzenesulfonic acid is prepared from the sulfonation of benzene using concentrated sulfuric acid: : This conversion illustrates aromatic sulfonation, which has been called "one of the most important reactions in industrial organic chemistry". Reactions Benzenesulfonic acid exhibits the reactions typical of other aromatic sulfonic acids, forming sulfonamides, sulfonyl chloride, and esters. The sulfonation is reversed above 220 °C. Dehydration with phosphorus pentoxide gives benzenesulfonic acid anhydride ((C6H5SO2)2O). Conversion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance (pharmacology), clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Ronald W. Estabrook, Estabrook, David Y. Cooper, Cooper, and Otto Rosenthal, Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of secondary metabolite, defensive compounds, fatty acids, and hormones. CYP enzymes have been identified in all kingdom (biology), kingdoms of life: animals, plants, fungus, fungi, protists, bacteria, and archaea, as well as in viruses. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. , more than 300,000 distinct CYP proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morpholine
Morpholine is an organic chemical compound having the chemical formula O( C H2CH2)2 NH. This heterocycle features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium. For example, treating morpholine with hydrochloric acid makes the salt morpholinium chloride. It is a colorless liquid with a weak, ammonia- or fish-like odor. The naming of morpholine is attributed to Ludwig Knorr, who incorrectly believed it to be part of the structure of morphine. Production Morpholine is often produced industrially by the dehydration of diethanolamine with sulfuric acid: : Uses Industrial applications Morpholine is a common additive, in parts per million concentrations, for pH adjustment in both fossil fuel and nuclear power plant steam systems. Morpholine is used because its volatility is about the same as water, so once it is added to the water, its concentration becomes distributed rather evenly in both the water a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Diimidazole
1,1'-Carbonyldiimidazole (CDI) is an organic compound with the molecular formula (C3H3N2)2CO. It is a white crystalline solid. It is often used for the coupling of amino acids for peptide synthesis and as a reagent in organic synthesis. Preparation CDI can be prepared straightforwardly by the reaction of phosgene with four equivalents of imidazole under anhydrous conditions. Removal of the side product, imidazolium chloride, and solvent results in the crystalline product in ~90% yield. :4 C3H4N2 + C(O)Cl2 → (C3H3N2)2CO + 2 3H3N2H2l In this conversion, the imidazole serves both as the nucleophile and the base. An alternative precursor 1-(trimethylsilyl)imidazole requires more preparative effort with the advantage that the coproduct trimethylsilyl chloride is volatile. CDI hydrolyzes readily to give back imidazole: :(C3H3N2)2CO + H2O → 2 C3H4N2 + CO2 The purity of CDI can be determined by the amount of CO2 that is formed upon hydrolysis. Use in synthesis CD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxybenzyl
Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl (Cbz or Z) group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water. The compound was first prepared by Leonidas Zervas in the early 1930s who used it for the introduction of the benzyloxycarbonyl protecting group, which became the basis of the Bergmann-Zervas carboxybenzyl method of peptide synthesis he developed with Max Bergmann. This was the first successful method of controlled peptide chemical synthesis and for twenty years it was the dominant procedure used worldwide until the 1950s. To this day, benzyl chloroformate is often used for amine group protection. Preparation The compound is prepared in the lab by treating benzyl alcohol with phosgene: : PhC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ugi Reaction
The Ugi reaction is a multi-component reaction in organic chemistry involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide. The reaction is named after Ivar Karl Ugi, who first reported this reaction in 1959. The Ugi reaction is exothermic and usually complete within minutes of adding the isocyanide. High concentration (0.5M - 2.0M) of reactants give the highest yields. Polar, aprotic solvents, like DMF, work well. However, methanol and ethanol have also been used successfully. This uncatalyzed reaction has an inherent high atom economy as only a molecule of water is lost, and the chemical yield in general is high. Several reviews have been published. Due to the reaction products being potential protein mimetics there have been many attempts to development an enantioselective Ugi reaction, the first successful report of which was in 2018. Reaction mechanism One plausible reaction mechanism is depicted below: Amine 1 and ketone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereoselective Synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric ( enantiomeric or diastereomeric) products in unequal amounts." Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer. Enantiomers are stereoisomers that have opposite configurations at every chiral center. Diastereomers are stereoisomers that differ at one or more chiral centers. Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity. Overview Many of the building blocks of biological systems such as sugars and amino acids are produced exclusive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Central Nervous System
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all parts of the bodies of bilaterally symmetric and triploblastic animals—that is, all multicellular animals except sponges and diploblasts. It is a structure composed of nervous tissue positioned along the rostral (nose end) to caudal (tail end) axis of the body and may have an enlarged section at the rostral end which is a brain. Only arthropods, cephalopods and vertebrates have a true brain (precursor structures exist in onychophorans, gastropods and lancelets). The rest of this article exclusively discusses the vertebrate central nervous system, which is radically distinct from all other animals. Overview In vertebrates, the brain and spinal cord are both enclosed in the meninges. The meninges provide a barrier to chemicals dissolv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intracerebroventricular Injection
Intracerebroventricular injection (also called ICV injection, i.c.v. injection, or sometimes ICVI) is an invasive injection technique of substances directly into the cerebrospinal fluid in cerebral ventricles in order to bypass the bloodbrain barrier. Although this barrier effectively protects the brain, it can prevent important medications from entering the CNS. The technique is widely used in biomedical research to introduce drugs, therapeutic RNAs, plasmid DNAs, and viral vectors into the CNS of diseased mice models. It can also be used in human in cases of neurodegenerative disorders like spinal muscular atrophy (SMA), or administering chemotherapy in gliomas as well as delivering neurotrophic factors to CNS. It can be contrasted with intraperitoneal injection as an alternative choice of route of administration with differing pharmacokinetic and pharmacodynamic effects. The Ommaya reservoir is a catheter system invented by Ayub Ommaya, a Pakistani neurosurgeon in 1963. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood–brain Barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that prevents solutes in the circulating blood from ''non-selectively'' crossing into the extracellular fluid of the central nervous system where neurons reside. The blood–brain barrier is formed by endothelial cells of the Capillary, capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive transport, passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function. The blood–brain barrier restricts the passage of pathogens, the diffusion of solutes in the blood, and Molecular mass, large or Hydrophile, hydrophilic molecules into the cerebrospinal fluid, while allowing the diffusion of Hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |