|
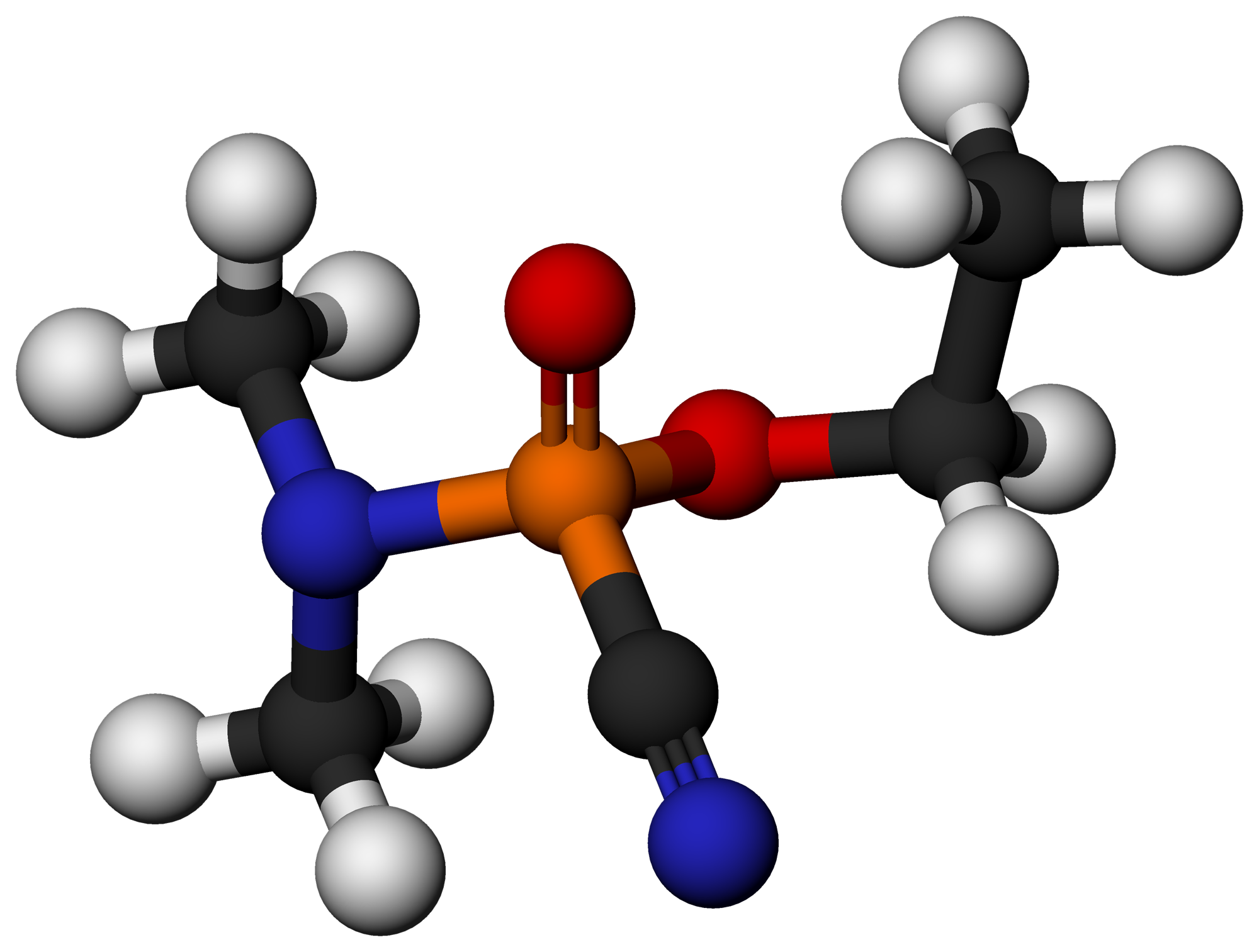
EA-4056
EA-4056 is a deadly carbamate nerve agent. It is lethal because it inhibits acetylcholinesterase. Inhibition causes an overly high accumulation of acetylcholine between the nerve and muscle cells. This paralyzes the muscles by preventing their relaxation. The paralyzed muscles includes the muscles used for breathing. Patent assigned to US Army for EA-4056 among other similar nerve agents was filed in December 7, 1967. Lethality Carbamates like EA-4056 are well absorbed by the lungs, gastrointestinal tracts, and the skin. Signs and symptoms from exposure to such carbamates are similar to other nerve agents. In general their penetration through the blood-brain barrier is difficult due to quaternary nitrogens in these molecules. Despite this, EA-4056 is claimed to be about three times more toxic than VX (another nerve agent). For VX, the median lethal dose (LD50) for 70 kg men via exposure to the skin is estimated to be 10 mg, and the lethal concentration tim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nerve Agent
Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that catalyzes the breakdown of acetylcholine, a neurotransmitter. Nerve agents are acetylcholinesterase inhibitors used as poison. Poisoning by a nerve agent leads to constriction of pupils, profuse salivation, convulsions, and involuntary urination and defecation, with the first symptoms appearing in seconds after exposure. Death by asphyxiation or cardiac arrest may follow in minutes due to the loss of the body's control over respiratory and other muscles. Some nerve agents are readily vaporized or aerosolized, and the primary portal of entry into the body is the respiratory system. Nerve agents can also be absorbed through the skin, requiring that those likely to be subjected to such agents wear a full body suit in addition to a respirato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
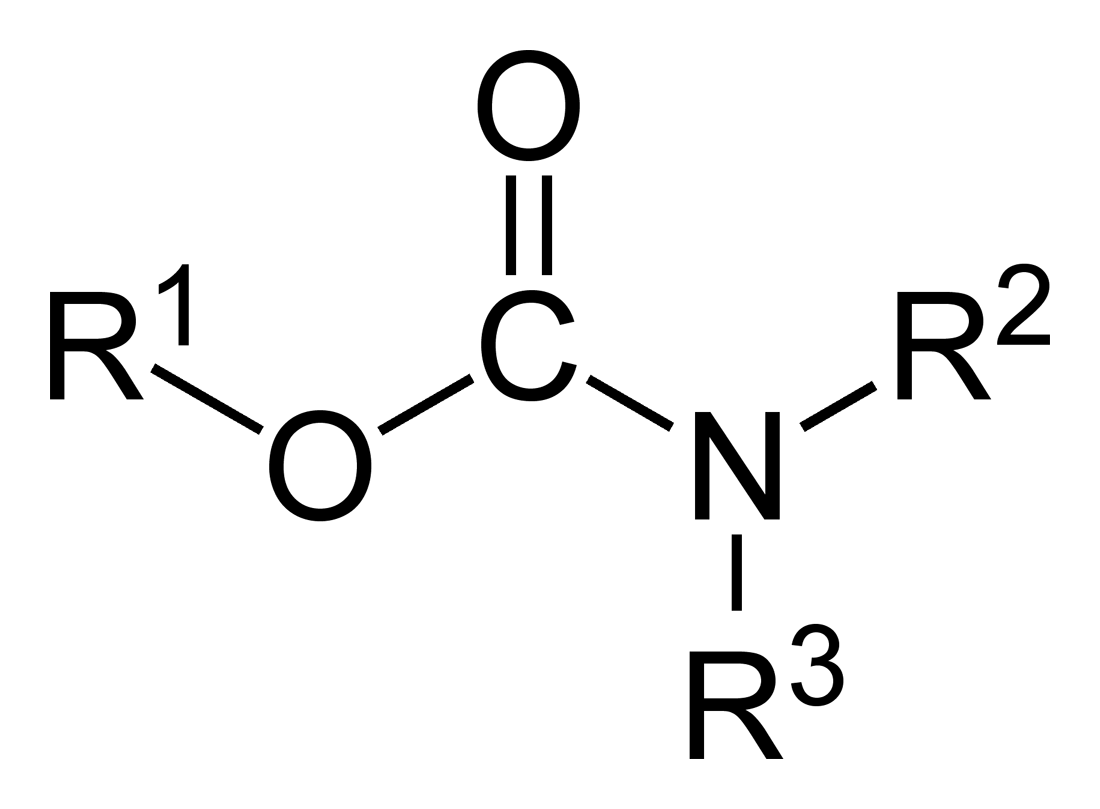
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonate immediatel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylcarbamoyl Chloride
Dimethylcarbamoyl chloride (DMCC) is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans, dimethylcarbamoyl chloride can only be used under stringent safety precautions. Production and occurrence The production of dimethylcarbamoyl chloride from phosgene and dimethylamine was reported as early as 1879 (reported as "Dimethylharnstoffchlorid" – dimethylurea chloride). : DMCC can be produced in high yields (90%) at 275 °C by reacting phosgene with gaseous dimethylamine in a flow reactor. To suppress the formation of ureas, excess phosgene is used (in a 3:1 ratio). The reaction can also be carried out at the laboratory scale with diphosgene or triphosgene and an aqueous dimethylamine solution in the two-phase system of benzene–xy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamylation
Isocyanic acid is a chemical compound with the structural formula HNCO, which is often written as . It is a colourless, volatile and poisonous substance, with a boiling point of 23.5 °C. It is the predominant tautomer of cyanic acid (). The derived anion of isocyanic acid is the same as the derived anion of cyanic acid, and that anion is , which is called cyanate. The related functional group is isocyanate; it is distinct from cyanate (), fulminate (), and nitrile oxide (). Isocyanic acid was discovered in 1830 by Justus von Liebig and Friedrich Wöhler. Isocyanic acid is the simplest stable chemical compound that contains carbon, hydrogen, nitrogen, and oxygen, the four most commonly found elements in organic chemistry and biology. It is the only fairly stable one of the four linear isomers with molecular formula HOCN that have been synthesized, the others being cyanic acid (cyanol, ) and the elusive fulminic acid () and isofulminic acid .William R. Martin and Dav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section Forms below), hence it is stored as an aqueous solution (formalin), which is also used to store animal specimens. It is the simplest of the aldehydes (). The common name of this substance comes from its similarity and relation to formic acid. Formaldehyde is an important precursor to many other materials and chemical compounds. In 1996, the installed capacity for the production of formaldehyde was estimated at 8.7 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Forms Formaldehyde is more complicated than many simple carbon compounds in that it adopts several diverse forms. These compounds can often be used interchangeably and can be interconverted. *Molecular formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: :2 CH3OH + NH3 → (CH3)2NH + 2 H2O Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mannich Reaction
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl () functional group by formaldehyde () and a primary or secondary amine () or ammonia (). The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich. center, 500px, Scheme 1 - Ammonia or an amine reacts with formaldehyde and an alpha acidic proton of a carbonyl compound to a beta amino carbonyl compound. The Mannich reaction starts with the nucleophilic addition of an amine to a carbonyl group followed by dehydration to the Schiff base. The Schiff base is an electrophile which reacts in a second step in an electrophilic addition with an enol formed from a carbonyl compound containing an acidic alpha-proton. The Mann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived from the Ancient Greek (bromos) meaning "stench", referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a native element in nature but it occurs in colourless soluble crystalline mineral halide salts, analogous to table salt. In fact, bromine and all the halogens are so reactive that they form bonds in pairs—never in single atoms. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its accumulation in the oceans. Commercial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present. Humidity depends on the temperature and pressure of the system of interest. The same amount of water vapor results in higher relative humidity in cool air than warm air. A related parameter is the dew point. The amount of water vapor needed to achieve saturation increases as the temperature increases. As the temperature of a parcel of air decreases it will eventually reach the saturation point without adding or losing water mass. The amount of water vapor contained within a parcel of air can vary significantly. For example, a parcel of air near saturation may contain 28 g of water per cubic metre of air at , but only 8 g of water per cubic metre of air at . Three primary measurements of humidity are widely employed: absolute, relative, and specific. Ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |