|
E226
Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula CaSO3·x(H2O). Two crystalline forms are known, the hemihydrate and the tetrahydrate, respectively CaSO3·½(H2O) and CaSO3·4(H2O). All forms are white solids. It is most notable as the product of flue-gas desulfurization. Production It is produced on a large scale by flue gas desulfurization (FGD). When coal or other fossil fuel is burned, the byproduct is known as flue gas. Flue gas often contains SO2, whose emission is often regulated to prevent acid rain. Sulfur dioxide is scrubbed before the remaining gases are emitted through the chimney stack. An economical way of scrubbing SO2 from flue gases is by treating the effluent with Ca(OH)2 hydrated lime or CaCO3 limestone. Scrubbing with limestone follows the following idealized reaction: : + → + Scrubbing with hydrated lime follows the following idealized reaction: : + → + The resulting calcium sulf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfites
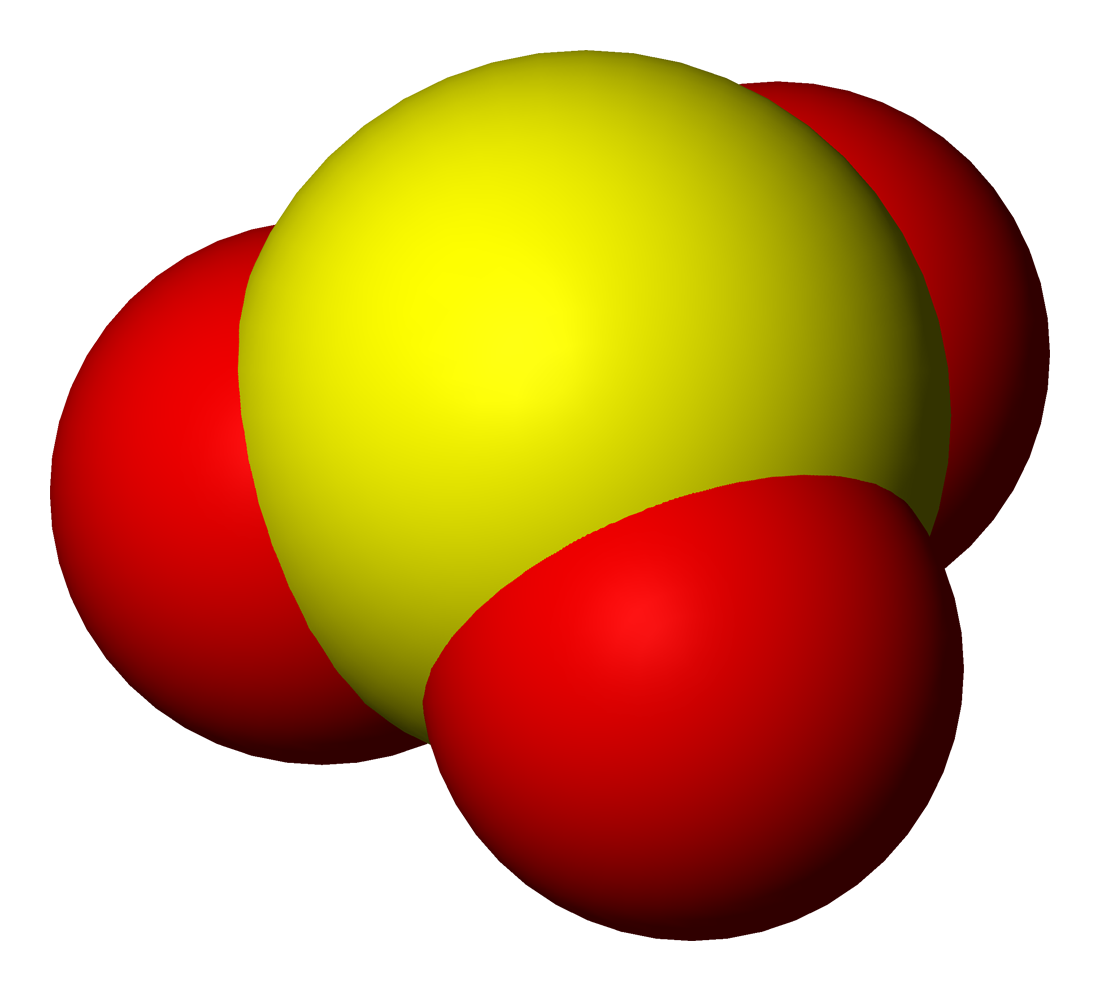
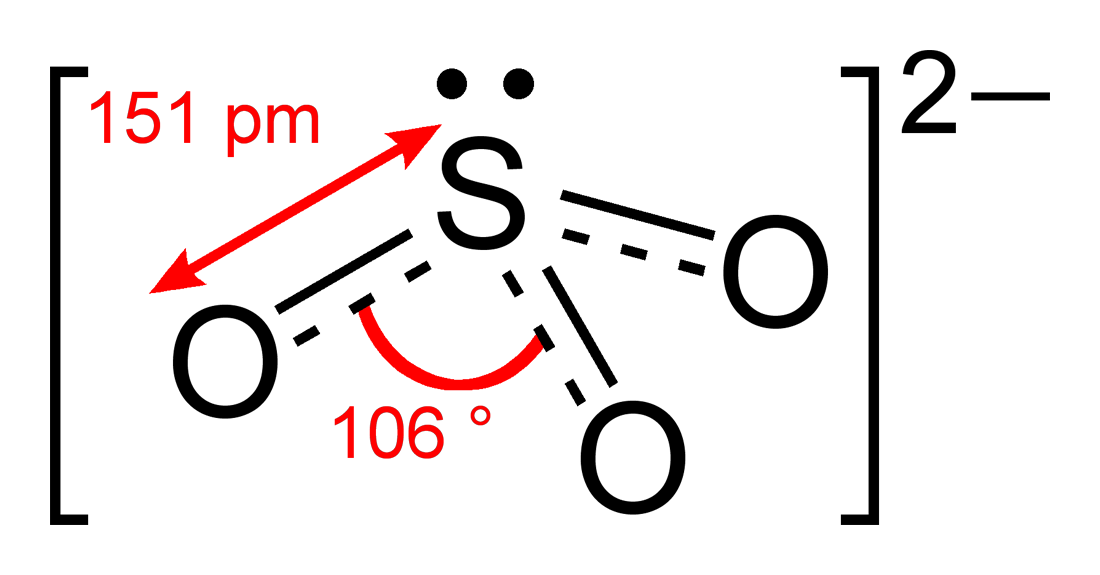
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfides
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide. Chemical properties The sulfide ion, S2−, does not exist in aqueous alkaline solutions of Na2S. Instead sulfide converts to hydrosulfide: :S2− + H2O → SH− + OH− Upon treatment with an acid, sulfide salts convert to hydrogen sulfide: :S2− + H+ → SH− :SH− + H+ → H2S Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides, polythionates, sulfite, or sulfate. Metal sulfides react with halogens, forming sulfur and metal salts. :8 MgS + 8 I2 → S8 + 8&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Compounds
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Campden Tablet
Campden tablets (potassium or sodium metabisulfite) are a sulphur-based product that is used primarily to sterilize wine, cider and in beer making to kill bacteria and to inhibit the growth of most wild yeast: this product is also used to eliminate both free chlorine and the more stable form, chloramine, from water solutions (e.g., drinking water from municipal sources). Campden tablets allow the amateur brewer to easily measure small quantities of sodium metabisulfite, so it can be used to protect against wild yeast and bacteria without affecting flavour. Untreated cider must frequently suffers from acetobacter contamination causing vinegar spoilage. Yeasts are resistant to the tablets but the acetobacter are easily killed off, hence treatment is important in cider production. Typical use is one crushed Campden tablet per US gallon (3.8 L) of must or wort. This dosage contributes 67 ppm sulfur dioxide to the wort but the level of active sulfur dioxide diminishes rapidly as it re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Bisulfite
Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. For properties of sodium bisulfite, refer to the table located to the right. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry. Synthesis Sodium bisulfite solutions can be prepared by treating a solution of suitable base, such as sodium hydroxide or sodium bicarbonate with sulfur dioxide. :SO2 + NaOH → NaHSO3 :SO2 + NaHCO3 → NaHSO3 + CO2 Attempts to crystallize the product yield Sodium me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Sulfite
Magnesium sulfite is the magnesium salt of sulfurous acid with the formula . Its most common hydrated form has 6 water molecules making it a hexahydrate, ·6. When heated above , it is dehydrated to magnesium sulfite trihydrate, or ·3. The anhydrous form is hygroscopic, meaning that it readily absorbs water from the air. See also *Calcium sulfite *Magnesium sulfate Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, ... (Epsom salt) References solubility tables of MgSO3 hydrates PDF H.D.Lutz, Dept. of Chemistry, University of Slegen.{{Magnesium compounds [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite Sulfate
A sulfite sulfate is a chemical compound that contains both sulfite and sulfate anions O3sup>2− O4sup>2−. These compounds were discovered in the 1980s as calcium and rare earth element salts. Minerals in this class were later discovered. Minerals may have sulfite as an essential component, or have it substituted for another anion as in alloriite. The related ions 3SOSO2sup>2− and O2SO)2SO2sup>2− may be produced in a reaction between sulfur dioxide and sulfate and exist in the solid form as tetramethyl ammonium salts. They have a significant partial pressure of sulfur dioxide. Related compounds are selenate selenites and tellurate tellurites with a varying chalcogen. They can be classed as mixed valent compounds. Production Europium and cerium rare earth sulfite sulfates are produced when heating the metal sulfite trihydrate in air. Ce2(SO3)3.3H2O + O2 → Ce2(SO3)2SO4 + 3H2O Ce2(SO3)3.3H2O + O2 → Ce2SO3(SO4)2 + 3H2O Other rare earth sulfite sulfates can be crys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2aq12.png)
2(SO3)N3-3D-balls.png)

