|
E224
Potassium metabisulfite, K2S2O5, also known as potassium pyrosulfite, is a white crystalline powder with a pungent odour. It is mainly used as an antioxidant or chemical sterilant. As a disulfite, it is chemically very similar to sodium metabisulfite, with which it is sometimes used interchangeably. Potassium metabisulfite has a monoclinic crystal structure. Preparation and reactions Potassium metabisulfite can be prepared by treating a solution of potassium hydroxide with sulfur dioxide. :2 SO2 + 2 KOH → K2S2O5 + H2O It decomposes at 190 °C, yielding potassium sulfite and sulfur dioxide: :K2S2O5 → K2SO3 + SO2 Uses It is used as a food additive, also known as E224. It is restricted in use and may cause allergic reactions in some sensitive persons. Wine Potassium metabisulfite is a common wine or must additive, in which it forms sulfur dioxide (SO2). Sulfur dioxide is a disinfectant. It also acts as a potent antioxidant, protecting both the color and d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfite
A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion . It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in water, these salts release the hydrogensulfite anion. These salts act equivalently to sodium hydrogensulfite or potassium hydrogensulfite. Structure In contrast to disulfate (), disulfite ion () has an unsymmetrical structure with an S-S bond. The oxidation state of the sulfur atom bonded to 3 oxygen atoms is +5 while oxidation number of other sulfur atom is +3. The anion consists of an SO2 group linked to an SO3 group, with the negative charge more localized on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å respectively. Production Salts of disulfite ion are produced by dehydration of salts of hydrogensulfite ion (). When solutions of sodium hydrogensulf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur- bearing fossil fuels. Structure and bonding SO2 is a bent molecule with ''C''2v symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke ''d'' orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. Occurrence Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. On other planets, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
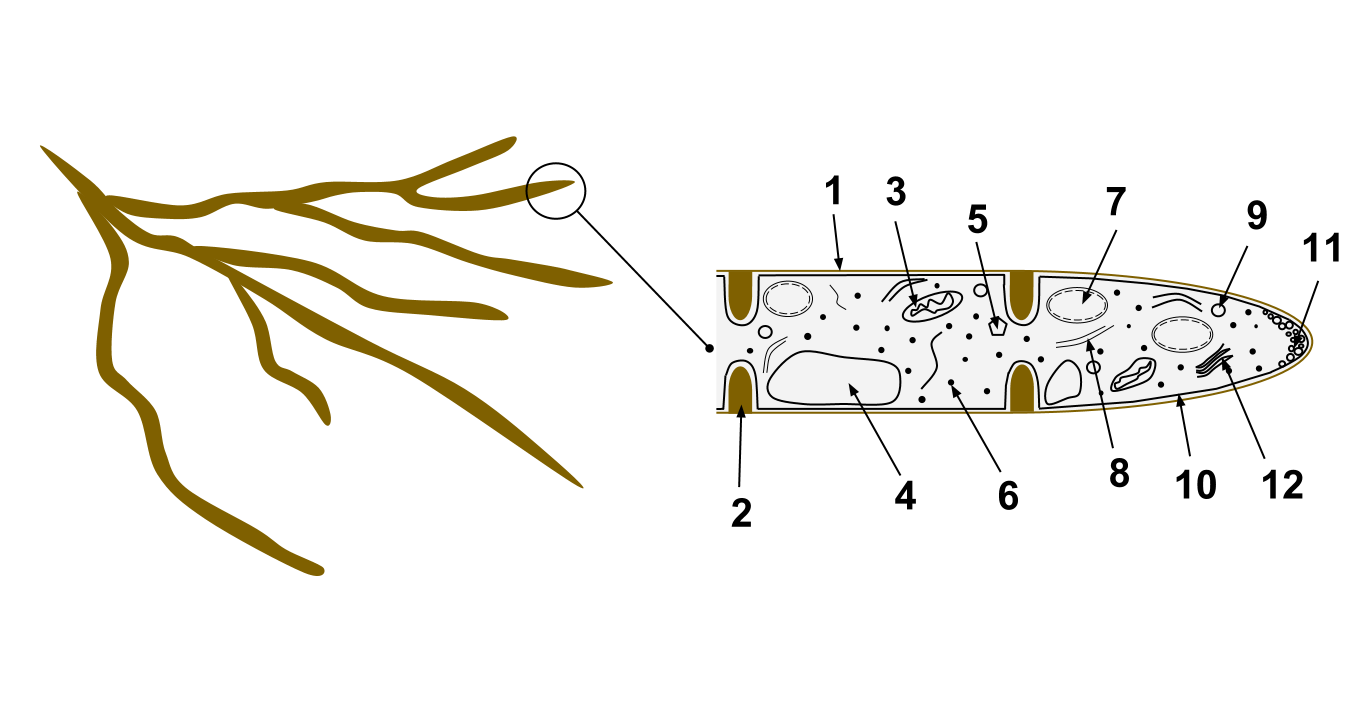
Fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from the other eukaryotic kingdoms, which by one traditional classification include Plantae, Animalia, Protozoa, and Chromista. A characteristic that places fungi in a different kingdom from plants, bacteria, and some protists is chitin in their cell walls. Fungi, like animals, are heterotrophs; they acquire their food by absorbing dissolved molecules, typically by secreting digestive enzymes into their environment. Fungi do not photosynthesize. Growth is their means of mobility, except for spores (a few of which are flagellated), which may travel through the air or water. Fungi are the principal decomposers in ecological systems. These and other differences place fungi in a single group of related organisms, named the ''Eumycota'' (''t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Compounds
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Campden Tablet
Campden tablets (potassium or sodium metabisulfite) are a sulphur-based product that is used primarily to sterilize wine, cider and in beer making to kill bacteria and to inhibit the growth of most wild yeast: this product is also used to eliminate both free chlorine and the more stable form, chloramine, from water solutions (e.g., drinking water from municipal sources). Campden tablets allow the amateur brewer to easily measure small quantities of sodium metabisulfite, so it can be used to protect against wild yeast and bacteria without affecting flavour. Untreated cider must frequently suffers from acetobacter contamination causing vinegar spoilage. Yeasts are resistant to the tablets but the acetobacter are easily killed off, hence treatment is important in cider production. Typical use is one crushed Campden tablet per US gallon (3.8 L) of must or wort. This dosage contributes 67 ppm sulfur dioxide to the wort but the level of active sulfur dioxide diminishes rapidly as it re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aam Papad
Aam papad is an Indian fruit leather made out of mango pulp mixed with concentrated sugar solution and sun dried. It is also known as ' ( Odia), ' ( Assamese), ' (Hindi), ' (Malayalam), ' (Telugu), ' (Bengali) and ' (Marathi). Traditional Aam Papad is sweet, although it is available in different varieties. It can be preserved for months making it popular in the off season of mangoes. Preparation Mango pulp is mixed with potassium metabisulfite and spread on trays to dry in the sun. After the first layer dries, another layer is spread over it and allowed to dry. The process is repeated until the desired thickness is reached. The thickness varies depending upon the quality of mango pulp used. When this thickness is reached the aam papad is cut into pieces and wrapped in oiled paper or into different packages. Aam Papad can be consumed in any season as it can be preserved for a long period of time. Aam Papad is manufactured in North India as well as South India. In the South I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Sulfite
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2 SO3. A white, water-soluble solid, it is used commercially as an antioxidant and preservative. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air. Preparation Sodium sulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide. When conducted in warm water, Na2SO3 initially precipitates as a white solid. With more SO2, the solid dissolves to give the disulfite, which crystallizes upon cooling. :SO2 + 2 NaOH -> Na2SO3 + H2O Sodium sulfite is made industrially by treating sulfur dioxide with a solution of sodium carbonate. The overall reaction is: :SO2 + Na2CO3 -> Na2SO3 + CO2 Applications Sodium sulfite is primarily used in the pulp and paper industry. It has been also applied in the thermomechanical conversion of wood to fibres (''defibration'') for producing medium density fibreboards (M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Preservative
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or by undesirable chemical changes. In general, preservation is implemented in two modes, chemical and physical. Chemical preservation entails adding chemical compounds to the product. Physical preservation entails processes such as refrigeration or drying.Erich Lück and Gert-Wolfhard von Rymon Lipinski "Foods, 3. Food Additives" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2002, Wiley-VCH, Weinheim. Preservative food additives reduce the risk of foodborne infections, decrease microbial spoilage, and preserve fresh attributes and nutritional quality. Some physical techniques for food preservation include dehydration, UV-C radiation, freeze-drying, and refrigeration. Chemical preservation and physical preservation techniques are som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lemon
The lemon (''Citrus limon'') is a species of small evergreen trees in the flowering plant family Rutaceae, native to Asia, primarily Northeast India (Assam), Northern Myanmar or China. The tree's ellipsoidal yellow fruit is used for culinary and non-culinary purposes throughout the world, primarily for its juice, which has both culinary and cleaning uses. The pulp and rind are also used in cooking and baking. The juice of the lemon is about 5% to 6% citric acid, with a pH of around 2.2, giving it a sour taste. The distinctive sour taste of lemon juice makes it a key ingredient in drinks and foods such as lemonade and lemon meringue pie. History The origin of the lemon is unknown, though lemons are thought to have first grown in Assam (a region in northeast India), northern Myanmar or China. A genomic study of the lemon indicated it was a hybrid between bitter orange (sour orange) and citron. Lemons are supposed to have entered Europe near southern Italy no later tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wort (brewing)
Wort () is the liquid extracted from the mashing process during the brewing of beer or whisky. Wort contains the sugars, the most important being maltose and maltotriose, that will be fermented by the brewing yeast to produce alcohol. Wort also contains crucial amino acids to provide nitrogen to the yeast as well as more complex proteins contributing to beer head retention and flavour. Production The first step in wort production is to obtain malt, which is made from dried, sprouted cereal grains, including barley. The malt is run through a mill, cracking the husk and exposing the starch inside. The milled grain is then mashed by mixing it with hot water, and then steeped, a process that enables enzymes to convert the starch in the malt into sugars which dissolve in the water. Sometimes the mash is heated at set intervals to alter the enzyme activity. The temperature of the mixture is usually increased to 78 °C (172 °F) for mashout. Lautering is the next st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |