|
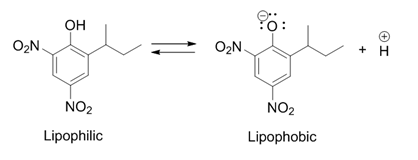
Dinoseb
Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity. It also finds use as a polymerisation inhibitor, where it is often referred to as DNBP. It is used to prevent the thermally induced polymerisation of styrene and other unsaturated monomers when they are being purified by distillation. History In 1892, dinitro-''ortho''-cresol (2,4-dinitro-6-methylphenol), a chemical compound closely related to dinoseb, was discovered in Germany and first used as an insecticide. It was later also used as an herbicide and also fungicide after those characteristics were discovered. In 1945 the ''ortho''-methyl group was replaced by a ''sec''-butyl group, producing dinoseb. This compound had a superior contact and stomach activity on insects and mites. Dinoseb b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinoseb Metabolism
Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity. It also finds use as a polymerisation inhibitor, where it is often referred to as DNBP. It is used to prevent the thermally induced polymerisation of styrene and other unsaturated monomers when they are being purified by distillation. History In 1892, dinitro-''ortho''-cresol (2,4-dinitro-6-methylphenol), a chemical compound closely related to dinoseb, was discovered in Germany and first used as an insecticide. It was later also used as an herbicide and also fungicide after those characteristics were discovered. In 1945 the ''ortho''-methyl group was replaced by a ''sec''-butyl group, producing dinoseb. This compound had a superior contact and stomach activity on insects and mites. Dinoseb b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uncoupler
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton-motive force, but the proton-motive force is dissipated before the ATP synthase can recapture this energy and use it to make ATP. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes. Description Classical uncouplers have five properties: # the complete release of respiratory control # the substitution of all coupled processes (ATP synthesis, transhydrogenation, reverse electron flow, active transport of cations, etc.) by a cyclic proton transport mediated by the uncoupler # the elimination of all protonic and cationic gradients generated across the mitochondrial or prokaryotic membrane # no discrimination in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrophenol
Dinitrophenols are chemical compounds which are nitro derivatives of phenol. There are six isomers of dinitrophenol: * 2,3-Dinitrophenol * 2,4-Dinitrophenol * 2,5-Dinitrophenol * 2,6-Dinitrophenol * 3,4-Dinitrophenol * 3,5-Dinitrophenol Dinitrophenols also form the core structure of some herbicides, which are collectively referred to as dinitrophenol herbicides, including: * Dinofenate * Dinoprop * Dinosam * Dinoseb * Dinoterb Dinoterb is a chemical compound used as an herbicide. It is an uncoupler An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobact ... * DNOC * Etinofen * Medinoterb {{Authority control ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerisation Inhibitor
Polymerisation inhibitors (US: polymerization inhibitors) are chemical compounds added to monomers to prevent their auto-polymerisation. Unsaturated monomers such as acrylates, vinyl chloride, butadiene and styrene require inhibitors for both processing and safe transport and storage. Many monomers are purified industrially by distillation, which can lead to thermally initiated polymerisation. Styrene for example is distilled at temperatures above 100 °C whereupon it undergoes thermal polymerisation at a rate of ~2% per hour. This polymerisation is undesirable, as it can foul the fractionating tower, it is also typically exothermic which can lead to a runaway reaction and potential explosion if left unchecked. Once initiated polymerisation is typically radical in mechanism and as such many polymerisation inhibitors act as radical scavengers. Inhibitors vs retarders The term 'inhibitor' is often used in a general sense to describe any compound used to prevent unwanted polymeri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz N. D. Kurie, Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (), which makes up 99% of all carbon on Earth; carbon-13 (), which makes up 1%; and carbon-14 (), which occurs in trace amounts, making up about 1 or 1.5 atoms per 1012 atoms of carbon in the atmosphere. Carbon-12 and carbon-13 are both stable, while carbon-14 is unstable and has a half-life of 5,730 ± 40 years. Carbon-14 decays into nitrogen-14 () through bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinamide Adenine Dinucleotide Phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a Cofactor (biochemistry), cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). It is used by all forms of cellular life. NADPH is the redox, reduced form of NADP. NADP differs from NAD+, NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine Moiety (chemistry), moiety. This extra phosphate is added by NAD+ kinase, NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+, NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase, NAD+ kinase adding the extra phosphate group. ADP-ribosyl cyclase allows for synthesis from nicotinamide in the salvage pathway, and NADP+ phosphatase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thylakoid Membrane 3
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana (singular: granum). Grana are connected by intergranal/stromal thylakoids, which join granum stacks together as a single functional compartment. In thylakoid membranes, chlorophyll pigments are found in packets called quantasomes. Each quantasome contains 230 to 250 chlorophyll molecules. Etymology The word ''Thylakoid'' comes from the Greek word ''thylakos'' or ''θύλακος'', meaning "sac" or "pouch". Thus, ''thylakoid'' means "sac-like" or "pouch-like". Structure Thylakoids are membrane-bound structures embedded in the chloroplast stroma. A stack of thylakoids is called a granum and resembles a stack of coins. Membrane The thylakoid membrane is the site of the light- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page for EPA reports on pesticide use ihere Selective herbicides control specific weed species, while leaving the desired crop relatively unharmed, while non-selective herbicides (sometimes called total weedkillers in commercial products) can be used to clear waste ground, industrial and construction sites, railways and railway embankments as they kill all plant material with which they come into contact. Apart from selective/non-selective, other important distinctions include ''persistence'' (also known as ''residual action'': how long the product stays in place and remains active), ''means of uptake'' (whether it is absorbed by above-ground foliage only, through the roots, or by other means), and ''mechanism of action'' (how it works). Historica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thin-layer Chromatography
Thin-layer chromatography (TLC) is a chromatography technique used to separate non-volatile mixtures. Thin-layer chromatography is performed on a sheet of an inert substrate such as glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide (alumina), or cellulose. This layer of adsorbent is known as the stationary phase. After the sample has been applied on the plate, a solvent or solvent mixture (known as the mobile phase) is drawn up the plate via capillary action. Because different analytes ascend the TLC plate at different rates, separation is achieved. It may be performed on the analytical scale as a means of monitoring the progress of a reaction, or on the preparative scale to purify small amounts of a compound. TLC is an analytical tool widely used because of its simplicity, relative low cost, high sensitivity, and speed of separation. TLC functions on the same principle as all chromatography: a com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATP Synthase
ATP synthase is a protein that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). It is classified under ligases as it changes ADP by the formation of P-O bond (phosphodiester bond). ATP synthase is a molecular machine. The overall reaction catalyzed by ATP synthase is: * ADP + Pi + 2H+out ATP + H2O + 2H+in The formation of ATP from ADP and Pi is energetically unfavorable and would normally proceed in the reverse direction. In order to drive this reaction forward, ATP synthase couples ATP synthesis during cellular respiration to an electrochemical gradient created by the difference in proton (H+) concentration across the inner mitochondrial membrane in eukaryotes or the plasma membrane in bacteria. During photosynthesis in plants, ATP is synthesized by ATP synthase using a proton gradient created in the thylakoid lumen through the thylakoid membrane and into the chloroplast stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |