|
Depsipeptides
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR, Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results in a decrease of H-bonding capability, which is responsible for secondary structure and folding patterns of peptides, thus inducing structural deformation of the helix and b-sheet structures. Because of decreased resonance delocalization in esters relative to amides, depsipeptides have lower rotational barriers for cis-trans isomerization and therefore they have more flexible structures than their native analogs. They are mainly found in marine and microbial natural products. Depsipeptide natural products 222px, Enterochelin is a depsipeptide that is an iron-transporter. Several depsipeptides have been found to exhibit anti-cancer properties. A depsipeptide enzyme inhibitor includes romidepsin, a member of the bicyclic peptide class, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depsipeptide Principle V
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR, Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results in a decrease of H-bonding capability, which is responsible for secondary structure and folding patterns of peptides, thus inducing structural deformation of the helix and b-sheet structures. Because of decreased resonance delocalization in esters relative to amides, depsipeptides have lower rotational barriers for cis-trans isomerization and therefore they have more flexible structures than their native analogs. They are mainly found in marine and microbial natural products. Depsipeptide natural products 222px, Enterochelin is a depsipeptide that is an iron-transporter. Several depsipeptides have been found to exhibit anti-cancer properties. A depsipeptide enzyme inhibitor includes romidepsin, a member of the bicyclic peptide class, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Papuamide
Papuamides A and B are depsipeptides which appear to protect T cells A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell re ... from HIV. They were isolated from the sponge Theonella, and are part of a larger group of structurally similar depsipeptides—also isolated from sponges—including neamphamide A, callipeltin, callipeltin A, and mirabamide, mirabamides A-D. References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passerini Reaction
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde (or ketone), and a carboxylic acid to form a α- acyloxy amide. This addition reaction is one of the oldest isocyanide-based multicomponent reactions (IMCR) and was first described in 1921 by Mario Passerini in Florence, Italy. It is typically carried out in aprotic solvents but can also be performed in ionic liquids such as water or Deep Eutectic solvents (DESs). It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in combinatorial and medicinal chemistry with recent utility in green chemistry and polymer chemistry. As isocyanides exhibit high functional group tolerance, chemoselectivity, regioselectivity, and stereoselectivity, the Passerini reaction has a wide range of synthetic applications.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 Mechanism The Passerini reaction has been hypothesiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mirabamide
Mirabamide A-D Mirabamides are sea sponge Sponges, the members of the phylum Porifera (; meaning 'pore bearer'), are a basal animal clade as a sister of the diploblasts. They are multicellular organisms that have bodies full of pores and channels allowing water to circulate throug ... isolates that inhibit HIV-1 fusion. References Depsipeptides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Callipeltin
Callipeltin A & B Callipeltin A and B are bio-active isolates of marine invertebrates. See also * Depsipeptide A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR, Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results ... * Papuamide References Depsipeptides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptomyces
''Streptomyces'' is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of ''Streptomyces'' bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin. Streptomycetes are characterised by a complex secondary metabolism. They produce over two-thirds of the clinically useful antibiotics of natural origin (e.g., neomycin, streptomycin, cypemycin, grisemycin, bottromycins and chloramphenicol). The antibiotic streptomycin takes its name directly from ''Streptomyces''. Streptomycetes are infrequent pathogens, though infections in humans, such as mycetoma, can be caused by '' S. somaliensis'' and '' S. sudanensis'', and in plants can be caused by '' S. cavi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important plastics l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
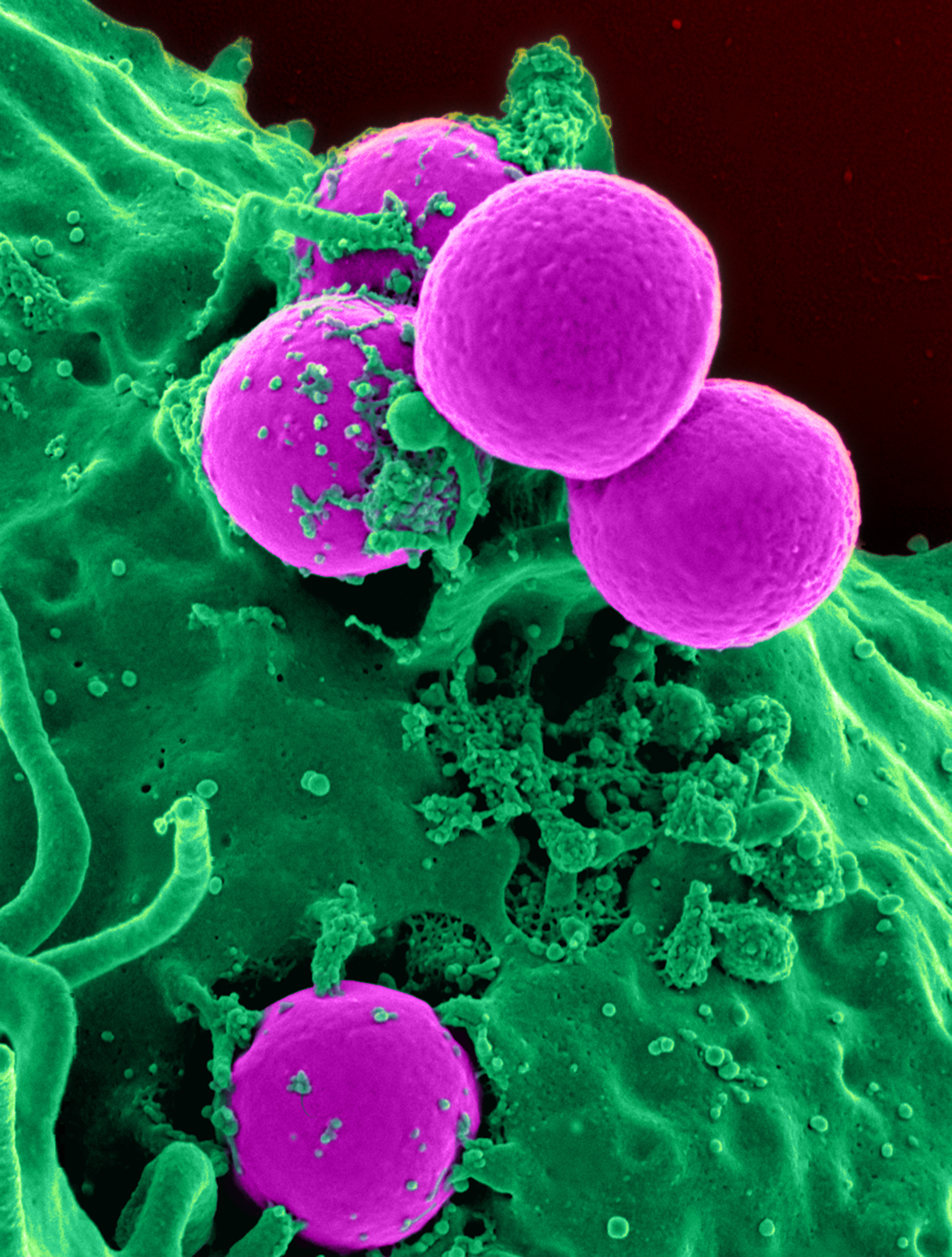
Methicillin-resistant Staphylococcus Aureus
Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of Gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths attributable to antimicrobial resistance in 2019. MRSA is any strain of ''S. aureus'' that has developed (through natural selection) or acquired (through horizontal gene transfer) a multiple drug resistance to beta-lactam antibiotics. Beta-lactam (β-lactam) antibiotics are a broad-spectrum group that include some penams (penicillin derivatives such as methicillin and oxacillin) and cephems such as the cephalosporins. Strains unable to resist these antibiotics are classified as methicillin-susceptible ''S. aureus'', or MSSA. MRSA is common in hospitals, prisons, and nursing homes, where people with open wounds, invasive devices such as catheters, and weakened immune systems are at greater risk of healt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |