Passerini Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde (or 
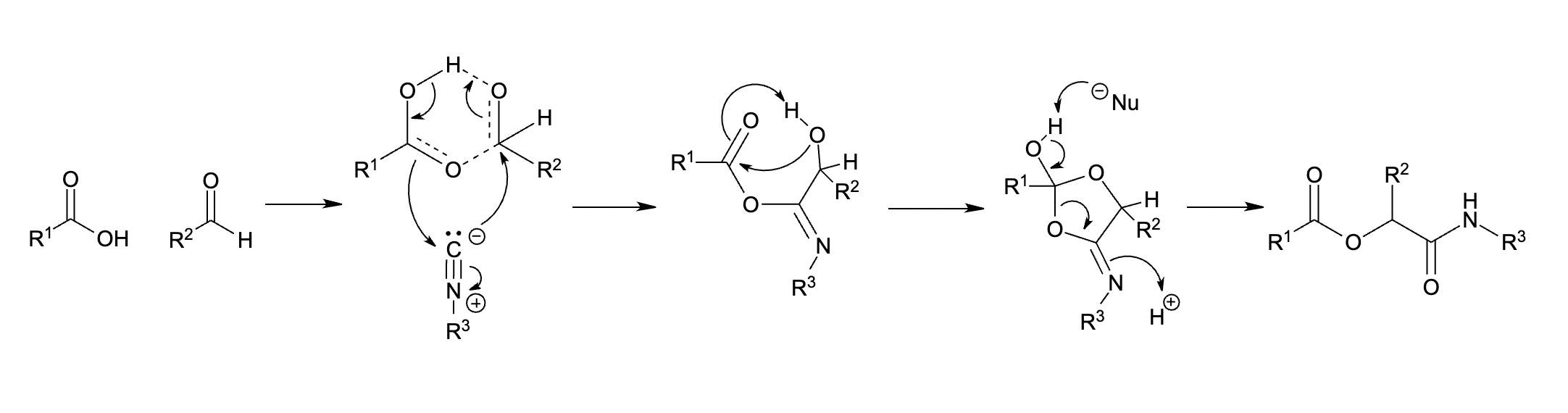 This mechanism involves a
This mechanism involves a
 In polar solvents, such as
In polar solvents, such as
 The original Passerini reaction produces acyclic
The original Passerini reaction produces acyclic
 This reaction has also been used for polymerization, monomer formation, and post-polymerization modification. The Passerini reaction has also been used to form sequence-defined polymers. Bifunctional substrates can be used to undergo post-polymerization modification or serve as precursors for polymerization.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 As this reaction has high functional group tolerance, the polymers created using this reaction are widely diverse with tuneable properties. Macromolecules that have been produced with this reaction include macroamides, macrocyclic depsipeptides, three-component
This reaction has also been used for polymerization, monomer formation, and post-polymerization modification. The Passerini reaction has also been used to form sequence-defined polymers. Bifunctional substrates can be used to undergo post-polymerization modification or serve as precursors for polymerization.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 As this reaction has high functional group tolerance, the polymers created using this reaction are widely diverse with tuneable properties. Macromolecules that have been produced with this reaction include macroamides, macrocyclic depsipeptides, three-component

ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
), and a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
to form a α- acyloxy amide. This addition reaction is one of the oldest isocyanide-based multicomponent reactions (IMCR) and was first described in 1921 by Mario Passerini in Florence, Italy. It is typically carried out in aprotic A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these solvents do not serve as proton donors in hydrogen bonding
In chemistry, a hydro ...
solvents but can also be performed in ionic liquids such as water or Deep Eutectic solvents (DESs). It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in combinatorial and medicinal chemistry with recent utility in green chemistry and polymer chemistry. As isocyanides exhibit high functional group tolerance, chemoselectivity, regioselectivity, and stereoselectivity, the Passerini reaction has a wide range of synthetic applications.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 Mechanism
The Passerini reaction has been hypothesized to occur through two mechanistic pathways.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 The reaction pathways are dependent on the solvent used.Concerted mechanism
A concerted mechanism, seen in SN2 and Diels−Alder reactions, is theorized to occur when the Passerini reagents are present at high concentration inaprotic A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these solvents do not serve as proton donors in hydrogen bonding
In chemistry, a hydro ...
solvents.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005
 This mechanism involves a
This mechanism involves a trimolecular reaction
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of stoichiometric coeffici ...
between the isocyanide, carboxylic acid, and carbonyl in a sequence of nucleophilic additions. The reaction proceeds first through an imidate
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between a carboximidic acid (R-C(=NR')OH) and an alcohol, with the general formula R-C(=NR')OR".
They are also known as imino ethers, sin ...
intermediate and then undergoes Mumm rearrangement The Mumm rearrangement is an organic reaction and a rearrangement reaction. It describes a 1,3(O-N) acyl transfer of an acyl imidate or isoimide group to an imide.
The reaction is of relevance as part of the Ugi reaction
The Ugi reaction is a m ...
to afford the Passerini product.
As the Mumm rearrangement The Mumm rearrangement is an organic reaction and a rearrangement reaction. It describes a 1,3(O-N) acyl transfer of an acyl imidate or isoimide group to an imide.
The reaction is of relevance as part of the Ugi reaction
The Ugi reaction is a m ...
requires a second carboxylic acid molecule, this mechanism classifies the Passerini reaction as an organocatalytic reaction.
Ionic mechanism
 In polar solvents, such as
In polar solvents, such as methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
or water, the carbonyl is protonated before nucleophilic addition of the isocyanide, affording a nitrilium ion intermediate. This is followed by the addition of a carboxylate, acyl group transfer and proton transfer respectively to give the desired Passerini product.
Reaction control
Molecular weights of polymers synthesized through the Passerini can be controlled through stoichiometric means. For example, polymer chain length and weight can adjusted through isocyanide stoichiometry, and polymer geometry can be influenced through starting reagents. To facilitate the Passerini reaction between bulky, sterically-hindered reagents, a vortex fluidic device can be used to induce high shear conditions. These conditions emulate the effects of high temperature and pressure, allowing the Passerini reaction to proceed fairly quickly. The Passerini reaction can also exhibit enantioselectivity. Addition of tert-butyl isocyanide to a wide variety of aldehydes (aromatic, heteroaromatic, olefinic, acetylenic, aliphatic) is achieved using a catalytic system of tetrachloride and a chiral bisphosphoramide which provides good yield and good enantioselectivities. For other types of isocyanides, rate of addition of isocyanide to reaction mixture dictates good yields and high selectivities.Applications
Apart from forming α- acyloxy amide products, the Passerini reaction can be used to form heterocycles, polymers,amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
, and medicinal products.
Heterocycles
depsipeptides A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR, Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results ...
which are labile in physiological conditions. To increase product stability for medicinal use, post-Passerini cyclization reactions have be used to afford heterocycles such as β-lactams, butenolides, and isocoumarins
Isocoumarin (1''H''-2-benzopyran-1-one; 3,4-benzo-2-pyrone) is a lactone, a type of natural organic compound.
Known natural compounds
* Thunberginol A and B
; dihydroisocoumarins
* Hydrangenol
* Phyllodulcin
* Thunberginol C, D, E and G
* T ...
. To enable these cyclizations, reagents are pre-functionalized with reactive groups (ex. halogens, azides, etc) and used in tandem with other reactions (ex. Passerini- Knoevenagel, Passerini- Dieckmann) to afford heterocyclic products. Compounds like three membered oxirane and aziridine derivatives, four-membered b-lactams
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words ''lactone'' + ''amide''.
Nomenclature
Greek prefixes in alphabetical order indicate ring size:
* α-Lactam (3-atom rings)
* β-Lacta ...
, and five-membered tetrasubstituted 4,5-dihydropyrazoles
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 ( ...
have been produced through this reaction.
Polymers
 This reaction has also been used for polymerization, monomer formation, and post-polymerization modification. The Passerini reaction has also been used to form sequence-defined polymers. Bifunctional substrates can be used to undergo post-polymerization modification or serve as precursors for polymerization.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 As this reaction has high functional group tolerance, the polymers created using this reaction are widely diverse with tuneable properties. Macromolecules that have been produced with this reaction include macroamides, macrocyclic depsipeptides, three-component
This reaction has also been used for polymerization, monomer formation, and post-polymerization modification. The Passerini reaction has also been used to form sequence-defined polymers. Bifunctional substrates can be used to undergo post-polymerization modification or serve as precursors for polymerization.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 As this reaction has high functional group tolerance, the polymers created using this reaction are widely diverse with tuneable properties. Macromolecules that have been produced with this reaction include macroamides, macrocyclic depsipeptides, three-component dendrimers
Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The ...
and three-armed star branched mesogen core molecules.
Amino acids and pharmaceuticals
Passerini reaction has been employed for the formation of structures like α-amino acids, α-hydroxy-β-amino acids, α-ketoamides, β-ketoamides, α-hydroxyketones
In organic chemistry a hydroxy ketone (often referred to simply as a ketol) is a functional group consisting of a ketone flanked by a hydroxyl group. In the two main classes, the hydroxyl group can be placed in the alpha position (an alpha-hydroxy ...
and α-aminoxyamides. The Passerini reaction has synthesized α-Acyloxy carboxamides that have demonstrated activity as anti-cancer medications along with functionalized 60 fullerenes used in medicinal and plant chemistry. This reaction has also been used as a synthetic step in the total synthesis of commercially available pharmaceuticals such as telaprevir (VX-950), an antiviral sold by Vertex Pharmaceuticals and Johnson & Johnson.
See also
* Ugi reactionReferences
{{Reflist Carbon-carbon bond forming reactions Multiple component reactions Name reactions Amide synthesis reactions