|
Dysfibrinogenemia
The dysfibrinogenemias consist of three types of fibrinogen disorders in which a critical blood clotting factor, fibrinogen, circulates at normal levels but is dysfunctional. Congenital dysfibrinogenemia is an inherited disorder in which one of the parental genes produces an abnormal fibrinogen. This fibrinogen interferes with normal blood clotting and/or lysis of blood clots. The condition therefore may cause pathological bleeding and/or thrombosis. Acquired dysfibrinogenemia is a non-hereditary disorder in which fibrinogen is dysfunctional due to the presence of liver disease, autoimmune disease, a plasma cell dyscrasias, or certain cancers. It is associated primarily with pathological bleeding. Hereditary fibrinogen Aα-Chain amyloidosis is a sub-category of congenital dysfibrinogenemia in which the dysfunctional fibrinogen does not cause bleeding or thrombosis but rather gradually accumulates in, and disrupts the function of, the kidney. Congenital dysfibrinogenemia is the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibrinogen
Fibrinogen (factor I) is a glycoprotein complex, produced in the liver, that circulates in the blood of all vertebrates. During tissue and vascular injury, it is converted enzymatically by thrombin to fibrin and then to a fibrin-based blood clot. Fibrin clots function primarily to occlude blood vessels to stop bleeding. Fibrin also binds and reduces the activity of thrombin. This activity, sometimes referred to as antithrombin I, limits clotting. Fibrin also mediates blood platelet and endothelial cell spreading, tissue fibroblast proliferation, capillary tube formation, and angiogenesis and thereby promotes revascularization and wound healing. Reduced and/or dysfunctional fibrinogens occur in various congenital and acquired human fibrinogen-related disorders. These disorders represent a group of rare conditions in which individuals may present with severe episodes of pathological bleeding and thrombosis; these conditions are treated by supplementing blood fibrinoge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypodysfibrinogenemia
Hypodysfibrinogenemia, also termed congenital hypodysfibrinogenemia, is a rare hereditary fibrinogen disorder cause by mutations in one or more of the genes that encode a factor critical for blood clotting, fibrinogen. These mutations result in the production and circulation at reduced levels of fibrinogen at least some of which is dysfunctional. Hypodysfibrinogenemia exhibits reduced penetrance, i.e. only some family members with the mutated gene develop symptoms. The disorder is similar to a form of dysfibrinogenemia termed congenital dysfibrinogenemia. However, congenital dysfibrinogenemia differs form hypodysfibrinogenemia in four ways. Congenital dysfibrinogenemia involves: the circulation at normal levels of fibrinogen at least some of which is dysfunctional; a different set of causative gene mutations; a somewhat different mix of clinical symptoms; and a much lower rate of penetrance. Hypodysfibrinogenemia causes episodes of pathological bleeding and thrombosis due not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
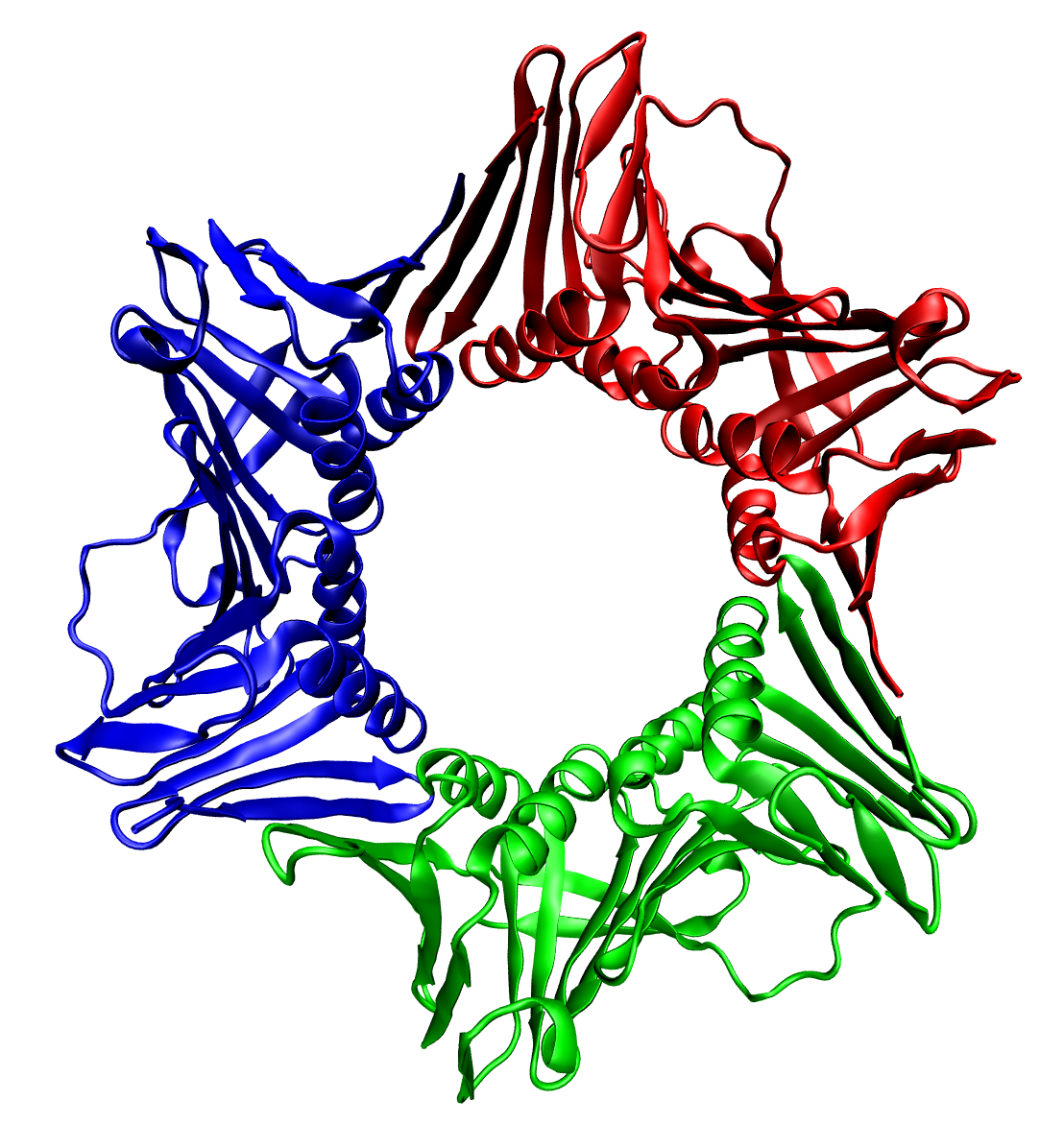
Protein Trimer
In biochemistry, a protein trimer is a macromolecular complex formed by three, usually non-covalently bound, macromolecules like proteins or nucleic acids. A homotrimer would be formed by three identical molecules. A heterotrimer would be formed by three different macromolecules. Type II Collagen is an example of homotrimeric protein. Porins usually arrange themselves in membranes as trimers. Bacteriophage T4 tail fiber Multiple copies of a polypeptide encoded by a gene often can form an aggregate referred to as a multimer. When a multimer is formed from polypeptides produced by two different mutant alleles of a particular gene, the mixed multimer may exhibit greater functional activity than the unmixed multimers formed by each of the mutants alone. When a mixed multimer displays increased functionality relative to the unmixed multimers, the phenomenon is referred to as intragenic complementation. The distal portion of each of the bacteriophage T4 tail fibers is encoded b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibrinopeptide B
The fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), are peptides which are located in the central region of the fibrous Fiber or fibre (from la, fibra, links=no) is a natural or artificial substance that is significantly longer than it is wide. Fibers are often used in the manufacture of other materials. The strongest engineering materials often incorpora ... glycoprotein fibrinogen (factor I) and are bond cleavage, cleaved by the enzyme thrombin (factor IIa) to convert fibrinogen into covalent bond, covalently-linked fibrin (factor IA) monomers. The N-terminus, N-terminal FpA is cleaved from the Fibrinogen alpha chain, Aα chains of fibrinogen and FpB from the Fibrinogen beta chain, Bβ chains of fibrinogen, with FpA released before FpB. Subsequent to their formation, fibrin monomers are converted to cross-linked fibrin polymers by the action of thrombin-activated factor XIII (fibrin stabilizing factor), and these fibrin polymers form the backbone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibrinopeptide A
The fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), are peptides which are located in the central region of the fibrous glycoprotein fibrinogen (factor I) and are cleaved by the enzyme thrombin (factor IIa) to convert fibrinogen into covalently-linked fibrin (factor IA) monomers. The N-terminal The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ... FpA is cleaved from the Aα chains of fibrinogen and FpB from the Bβ chains of fibrinogen, with FpA released before FpB. Subsequent to their formation, fibrin monomers are converted to cross-linked fibrin polymers by the action of thrombin-activated factor XIII (fibrin stabilizing factor), and these fibrin polymers form the backbone of a thrombus (blood clot). Hence, the fibrinopeptides are sensitive markers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thrombin
Thrombin (, ''fibrinogenase'', ''thrombase'', ''thrombofort'', ''topical'', ''thrombin-C'', ''tropostasin'', ''activated blood-coagulation factor II'', ''blood-coagulation factor IIa'', ''factor IIa'', ''E thrombin'', ''beta-thrombin'', ''gamma-thrombin'') is a serine protease, an enzyme that, in humans, is encoded by the ''F2'' gene. Prothrombin (coagulation factor II) is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions. History After the description of fibrinogen and fibrin, Alexander Schmidt hypothesised the existence of an enzyme that converts fibrinogen into fibrin in 1872. Prothrombin was discovered by Pekelharing in 1894. Physiology Synthesis Thrombin is produced by the enzymatic cleavage of two sites on prothrombin by activated Factor X (Xa). The activity of factor Xa is greatly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platelets
Platelets, also called thrombocytes (from Greek θρόμβος, "clot" and κύτος, "cell"), are a component of blood whose function (along with the coagulation factors) is to react to bleeding from blood vessel injury by clumping, thereby initiating a blood clot. Platelets have no cell nucleus; they are fragments of cytoplasm that are derived from the megakaryocytes of the bone marrow or lung, which then enter the circulation. Platelets are found only in mammals, whereas in other vertebrates (e.g. birds, amphibians), thrombocytes circulate as intact mononuclear cells. One major function of platelets is to contribute to hemostasis: the process of stopping bleeding at the site of interrupted endothelium. They gather at the site and, unless the interruption is physically too large, they plug the hole. First, platelets attach to substances outside the interrupted endothelium: ''adhesion''. Second, they change shape, turn on receptors and secrete chemical messengers: ''ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sialylation
Sialic acids are a class of alpha-keto acid sugars with a nine- carbon backbone. The term "sialic acid" (from the Greek for saliva, - ''síalon'') was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this group is ''N''-acetylneuraminic acid (Neu5Ac or NANA) found in animals and some prokaryotes. Sialic acids are found widely distributed in animal tissues and related forms are found to a lesser extent in other organisms like in some micro-algae, bacteria and archaea. Sialic acids are commonly part of glycoproteins, glycolipids or gangliosides, where they decorate the end of sugar chains at the surface of cells or soluble proteins. However, sialic acids have been also observed in ''Drosophila'' embryos and other insects. Generally, plants seem not to contain or display sialic acids. In humans the brain has the highest sialic acid content, where these acids play an important role in neural transmission and ganglioside structure in synapt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylation
Glycosylation is the reaction in which a carbohydrate (or 'glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not always in chemistry), glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation (also 'non-enzymatic glycation' and 'non-enzymatic glycosylation') may refer to a non-enzymatic reaction (though in practice, 'glycation' often refers more specifically to Maillard-type reactions). Glycosylation is a form of co-translational and post-translational modification. Glycans serve a variety of structural and functional roles in membrane and secreted proteins. The majority of proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as the ''O''-GlcNAc modification. Aglycosylation is a feature of engineered antibodies to bypass glycosylation. Fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sialic Acid
Sialic acids are a class of alpha-keto acid sugars with a nine-carbon backbone. The term "sialic acid" (from the Greek for saliva, - ''síalon'') was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this group is ''N''-acetylneuraminic acid (Neu5Ac or NANA) found in animals and some prokaryotes. Sialic acids are found widely distributed in animal tissues and related forms are found to a lesser extent in other organisms like in some micro-algae, bacteria and archaea. Sialic acids are commonly part of glycoproteins, glycolipids or gangliosides, where they decorate the end of sugar chains at the surface of cells or soluble proteins. However, sialic acids have been also observed in ''Drosophila'' embryos and other insects. Generally, plants seem not to contain or display sialic acids. In humans the brain has the highest sialic acid content, where these acids play an important role in neural transmission and ganglioside structure in synapto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars ( monosaccharides, or oligosaccharides). They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as cellulose and chitin. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |