|
DuPhos
DuPhos is a class of organophosphorus compound that are used ligands for asymmetric synthesis. The name DuPhos is derived from (1) the chemical company that sponsored the research leading to this ligand's invention, DuPont and (2) the compound is a diphosphine ligand type. Specifically it is classified as a C2-symmetric ligand, consisting of two phospholanes rings affixed to a benzene ring. The ligand was introduced in 1991 by M.J. Burk and first demonstrated in asymmetric hydrogenation of certain enamide esters to amino acid precursors: : Other chiral diphosphine ligands were known at the time of invention, e.g. DIOP, DIPAMP, CHIRAPHOS, but DuPhos was found to be more effective. Description The ligand consists of two 2,5-alkyl-substituted phospholane rings connected by a 1,2-phenyl bridge. The alkyl group can be methyl, ethyl, propyl, or isopropyl. In the closely related bis(dimethylphospholano)ethane or BPE ligand the ''o''-phenylene bridge is replaced by a 1,2-ethylene bridg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DuPhos Ligands
DuPhos is a class of organophosphorus compound that are used ligands for asymmetric synthesis. The name DuPhos is derived from (1) the chemical company that sponsored the research leading to this ligand's invention, DuPont and (2) the compound is a diphosphine ligand type. Specifically it is classified as a C2-Symmetric ligands, C2-symmetric ligand, consisting of two phospholanes rings affixed to a benzene ring. The ligand was introduced in 1991 by M.J. Burk and first demonstrated in asymmetric hydrogenation of certain enamide esters to amino acid precursors: : Other chiral diphosphine ligands were known at the time of invention, e.g. DIOP, DIPAMP, CHIRAPHOS, but DuPhos was found to be more effective. Description The ligand consists of two 2,5-alkyl-substituted phospholane rings connected by a 1,2-phenyl bridge. The alkyl group can be methyl, ethyl group, ethyl, propyl, or isopropyl. In the closely related bis(dimethylphospholano)ethane or BPE ligand the ''o''-phenylene bridge i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
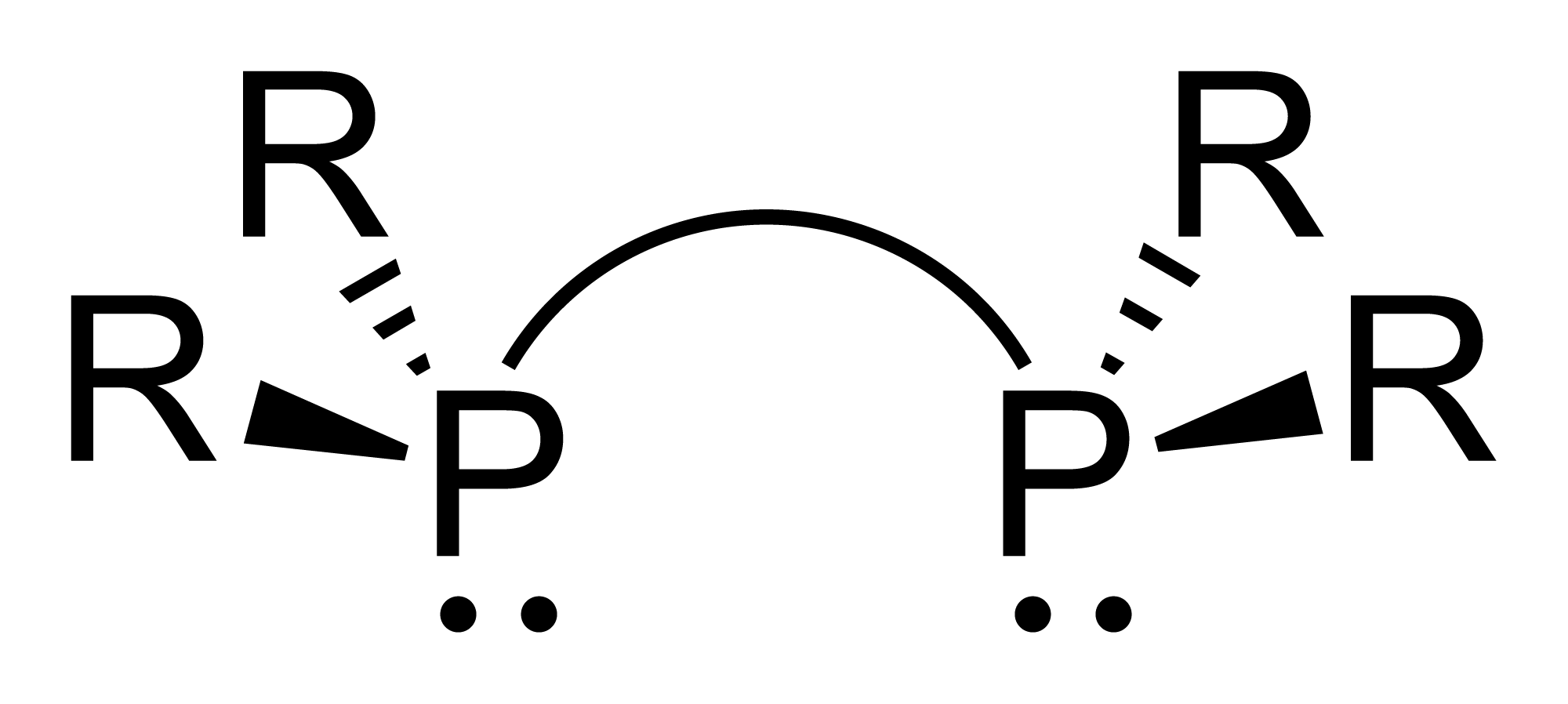
Diphosphine Ligand
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligand, phosphine ligands in inorganic chemistry, inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelate, chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in Homogeneous catalysis, homogeneous catalysts. Synthesis image:IPr2PCl.png, 222px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C2-Symmetric Ligands
In homogeneous catalysis, ''C''2-symmetric ligands refer to ligands that lack mirror symmetry but have ''C''2 symmetry (two-fold rotational symmetry). Such ligands are usually bidentate and are valuable in catalysis. The ''C''2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. ''C''2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including ''C''2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product. Examples An early ''C''2-symmetric ligand, diphosphine catalytic ligand DIPAMP, was developed in 1968 by William S. Knowles and coworkers of Monsanto Company, who shared the 2001 Nobel Prize in Chemistry. This ligand was used in the industrial production of -DOPA. : Some classes of ''C''2-symmetric li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholane
Phospholane is the organophosphorus compound with the formula (CH2)4PH. This colorless liquid is the parent member of a family of five-membered, saturated rings containing phosphorus. Although phospholane itself is only of minor academic interest, the class of C- and P-substituted phospholanes are valued ligands in asymmetric hydrogenation and related areas of homogeneous catalysis. Phospholane is prepared by reduction of 1-chlorophospholane, which in turn is obtained by the reaction of 1-phenylphospholane and phosphorus trichloride Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic a .... {{clear References Phosphorus heterocycles Five-membered rings ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone. Uses Benzophenone can be used as a photo initiator in UV(Ultra-violet)-curing applications such as inks, imaging, and clear coatings in the printing industry. Benzophenone prevents ultraviolet ( UV) light from damaging scents and colors in products such as perfumes and soaps. Benzophenone can also be added to plastic packaging as a UV blocker to prevent photo-degradation of the packaging polymers or its contents. Its use allows manufacturers to package the product in clear glass or plastic (such as a PETE water bottle). Without it, opaque or dark packaging would be required. In biological applications, benzophenones have been used extensively as photophysical probes to identify and map peptide–protein interactions. Benzophenone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Conformation
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations, conformations that correspond to local minima on the potential energy surface are specifically called conformational isomers or conformers. Conformations that correspond to local maxima on the energy surface are the transition states between the local-minimum conformational isomers. Rotations about single bonds involve overcoming a rotational energy barrier to interconvert one conformer to another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of multiple conformers; if the energy barrier is high enough then there is restricted rotation, a molecule may exist for a rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductive Amination
Reductive amination (also known as reductive alkylation) is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered the most important way to make amines, and a majority of amines made in the pharmaceutical industry are made this way. Reaction process In this organic reaction, the amine first reacts with the carbonyl group to form a hemiaminal species, which subsequently loses one molecule of water in a reversible manner by alkylimino-de-oxo-bisubstitution, to form the imine. The equilibrium between aldehyde/ketone and imine can be shifted toward imine formation by removal of the formed water through physical or chemical means. This intermediate imine can then be isolated and reduced with a suitable reducing agent (e.g., sodium borohydride). This method is sometimes called indirect reductive amination. In a separate approach, imine formation and redu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. Synthesis Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones. : Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides. Uses Hydrazones are the basis for various analyses of ketones and aldehydes. For example, dinitrophenylhydrazine coated onto a silica sorbent is the basis of an adsorption cartridge. The hydrazones are then eluted and analyzed by HPLC using a UV detector. The compound carbonyl cyanide-''p''-trifluoromethoxyphenylhydrazone (abbreviated as FCCP) is used to uncouple ATP synthesis and reduction of oxygen in oxidative phosphorylation in molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoyl Chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula . It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring () with an acyl chloride () substituent. It is mainly useful for the production of peroxides but is generally useful in other areas such as in the preparation of dyes, perfumes, pharmaceuticals, and resins. Preparation Benzoyl chloride is produced from benzotrichloride using either water or benzoic acid: :C6H5CCl3 + H2O -> C6H5COCl + 2 HCl :C6H5CCl3 + C6H5CO2H -> 2 C6H5COCl + HCl As with other acyl chlorides, it can be generated from the parent acid and standard chlorinating agents such as phosphorus pentachloride, thionyl chloride, and oxalyl chloride. It was first prepared by treatment of benzaldehyde with chlorine. An early method for production of benzoyl chloride involved chlorination of benzyl alcohol. Reactions It reacts with water to produce hydrochloric acid and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |